User:Milica Nenadovich/Sandbox 1
From Proteopedia
| Line 39: | Line 39: | ||
====Active Site==== | ====Active Site==== | ||
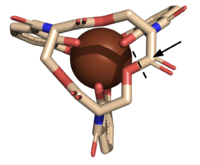
| - | The <scene name='10/1075192/Active_site/5'>active site</scene> of Syn-F4 lies within the central hole of the structure, made up of five catalytic residues (E26, H74, R77, K78, and R85) that facilitate binding to ferric enterobactin and catalyze hydrolysis of the scissile (ester) bond, which is indicated by the dotted line ('''Fig. 5''') <ref name="Kurihara"/>. The mutation of any of these five | + | The <scene name='10/1075192/Active_site/5'>active site</scene> of Syn-F4 lies within the central hole of the structure, made up of five catalytic residues (E26, H74, R77, K78, and R85) that facilitate binding to ferric enterobactin and catalyze hydrolysis of the scissile (ester) bond, which is indicated by the dotted line ('''Fig. 5''') <ref name="Kurihara"/>. The mutation of any of these five residues completely abrogates the function of the enzyme <ref name="Kurihara"/>. |
====Mechanism==== | ====Mechanism==== | ||
Current revision
Syn-F4, a de novo Ferric Enterobactin Esterase
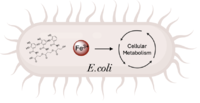
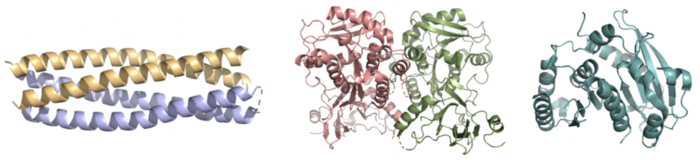
IntroductionBackgroundis a de novo ferric enterobactin esterase designed to highlight the potential of synthetic biology in creating simpler protein structures capable of performing complex functions [1]. Syn-F4 is also the first de novo protein catalytically active in vitro and biologically functional in vivo [1]. Ferric enterobactin esterases are a type of endogenous bacterial enzyme that break ester bonds of small metabolites bound to iron, allowing iron to be released into and utilized by bacterial cells (Fig. 1) [2] [3]. Iron plays crucial roles in various physiological processes in bacteria, including cellular respiration, oxidative stress responses, and DNA and protein synthesis (Fig. 1) [3]. is one such native esterase responsible for releasing Fe3+ from iron-chelating compounds, such as ferric enterobactin, within the cell, making it critical for bacterial survival [2] [4]. In order to mimic evolutionary selection of proteins for specific biological functions, Syn-IF was a de novo bifunctional protein designed to perform iron release, similar to Fes. Syn-IF was subjected to a series of random mutagenesis to individually select for its iron-releasing function, leading to the creation of Syn-F4 [5]. While Syn-F4 catalysis is 1000-fold slower than that of a native Fes enzyme, it is still sufficient in rescuing function in ∆fes E.coli [1] [5]. Whereas most ferric enterobactin esterases are serine hydrolases that function via a catalytic triad, such as Fes, Syn-F4 performs its function via a catalytic dyad without the involvement of a serine active site residue (Fig. 2) [1] [4]. One other serine hydrolase, , also performs catalysis through a catalytic dyad but still uses a serine residue as the nucleophile (Fig. 2) [4] [6]. The unassuming design of Syn-F4 was modeled after the de novo protein , which was originally designed to be used as a "building block" for other synthetic proteins and nanotechnology designs [7]. The crystallization of WA20 reveals an alpha-helix bundle structure, which served as the basis for the design of Syn-F4 [1] [7] . LigandFerric enterobactin is a metabolite that is part of a larger family of siderophores, which are low-molecular-weight, high-affinity, iron-chelating compounds that facilitate the transport of iron across the cell membrane [2] [3]. (FeEnt) is characterized by three catecholamine groups linked together by a trilactone ring and an exceptionally high affinity for Fe3+ (Kd = 10−52 M) [2] [8]. It is easily degraded when not bound to iron, which is coordinated to FeEnt by six hydroxyl groups and released by the hydrolysis of an ester bond [2] [8]. StructureAlpha-Helix BundleThe crystallization of the de novo protein, WA20, revealed an homodimer structure, which served as the foundation for the design of Syn-F4 [7]. While structurally similar, Syn-F4 features a novel active site characterized by the presence of a [1]. The symmetry of the four-helix bundle, combined with the configuration of the central hole, facilitates catalytic activity by effectively cleaving the ester bond in ferric enterobactin [1].
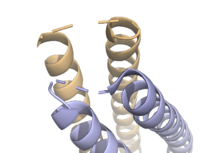
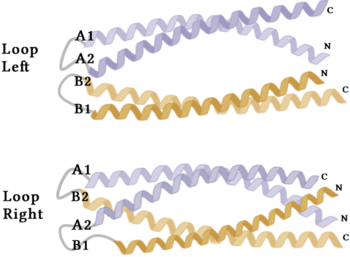
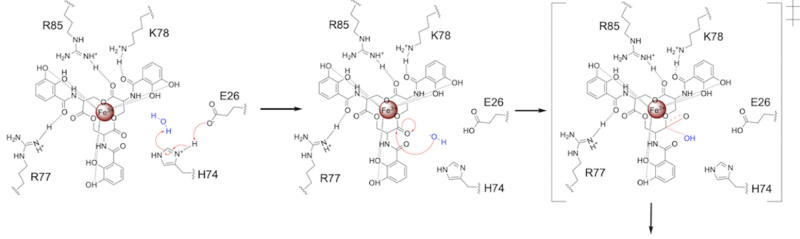
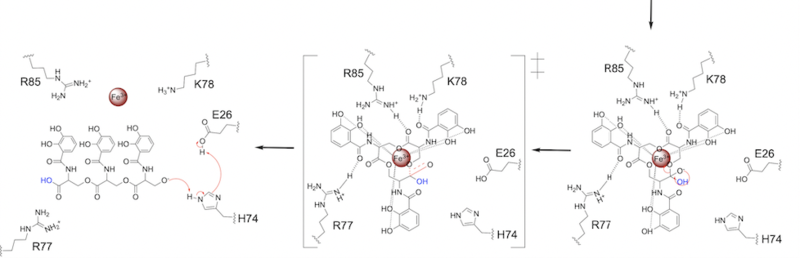
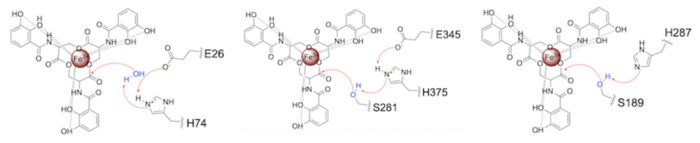
Stabilizing FactorsThe on either side of the bundles largely contribute to the stability of Syn-F4. Due to the symmetry of the chains, both chain A and chain B display the same residues and are linked by unresolved residues (Fig. 3) [1]. The accumulated negative charge on the C-termini is stabilized by positively-charged residues (H10, H45, H46, R102), and conversely, the accumulated positive charge on the N-termini is stabilized by negatively-charged residues (D59). Additionally, the within the core of Syn-F4 interact via Van der Waals to promote association between the helices. The orientation of the alpha helices and their associated interactions could not be definitively determined due to the absence of loop residues in the crystallized structure of Syn-F4 (Fig. 3) [1]. To address this, molecular dynamics (MD) simulations were performed to estimate the predominant conformation of the helices [1]. Two potential conformations were identified: "loop left" and "loop right" (Fig. 4) [1]. Among these, the "loop left" conformation demonstrated greater stability and was therefore considered the preferred structure (Fig. 4) [1]. FunctionActive SiteThe of Syn-F4 lies within the central hole of the structure, made up of five catalytic residues (E26, H74, R77, K78, and R85) that facilitate binding to ferric enterobactin and catalyze hydrolysis of the scissile (ester) bond, which is indicated by the dotted line (Fig. 5) [1]. The mutation of any of these five residues completely abrogates the function of the enzyme [1]. MechanismThe mechanism of Syn-F4 was proposed to function at pH 6.0 via a catalytic dyad with histidine (H74) and glutamate (E26) residues and water attacking as the nucleophile [1]. The electrophile is a carbonyl carbon involved in one of the ester bonds of the ligand, denoted by an arrow (Fig. 5). A representation of the possible mechanism of Syn-F4 performing hydrolysis of the scissile bond of ferric enterobactin follows: Step 1E26 first deprotonates H74, which then deprotonates H2O in turn (Fig. 6). The newly-formed hydroxide attacks the electrophile, kicking electron density up onto the carbonyl oxygen in the first transition state (Fig. 6). Step 2The second transition state is characterized by the electron density on the carbonyl oxygen collapsing and kicking off the alcohol leaving group (Fig. 7). As the ferric enterobactin structure linearizes, Fe3+ is released, and the enzyme is reset by the re-protonation of H74 by E26 (Fig. 7). Related EnzymesBoth the structure and mechanism of Syn-F4 differ from other native ferric enterobactin esterases whose structures have been previously determined. Native Fes and IroE enzymes are both serine hydrolases, which means a serine residue acts as their nucleophile [4] [6] [9]. Native is a homodimer that acts via a catalytic triad (S281, E345, and H375), whereas acts via a catalytic dyad (S189 and H287), similar to Syn-F4 (Fig. 8) [4] [6] [9]. RelevanceSynthetic BiologyDe novo proteins are at the forefront of biochemical and industrial applications, designed to mimic the evolutionary selection of proteins for highly specific functions. These engineered proteins show significant promise in enhancing antibiotic therapies, particularly in light of rising antibiotic resistance. For example, targeting bacterial iron acquisition pathways may offer an effective alternative to traditional antibiotic treatments [10] [11]. Furthermore, as our understanding of cellular signaling pathways advances, de novo proteins present new opportunities for cancer therapy. Through synthetic biology, it is possible to increase drug efficacy by designing agents that selectively target malignant cells while preserving healthy tissue [12]. Additionally, programming bacteria through synthetic compounds could enable bacteriotherapy approaches, in which engineered bacteria express specific proteins that bind to tumor-associated antigen markers, offering a novel strategy for cancer targeting and treatment [13]. Synthetic biology holds vast potential across a multitude of fields, with applications that span far beyond initial application. Its development is set to become integral to the future of biomedical research and innovation. As the field of synthetic biology continues to advance, its potential applications appear boundless [14] [15]. References
| ||||||||||||
Student Contributors
- Milica Nenadovich
- Reesha Bhagat