We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox323
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
=== General Structure and Origins === | === General Structure and Origins === | ||
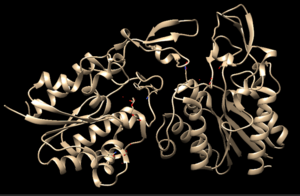
| - | 4Q7Q exists as a homodimer Quaternary structure.<ref name="Sequence">4Q7Q. Protein Database, 2014. https://www.rcsb.org/structure/4Q7Q</ref> Analyzing primary and Quaternary structures of 4Q7Q with SPRITE | + | 4Q7Q exists as a homodimer Quaternary structure.<ref name="Sequence">4Q7Q. Protein Database, 2014. https://www.rcsb.org/structure/4Q7Q</ref> Analyzing primary and Quaternary structures of 4Q7Q with SPRITE revealed two chains identical in both shape and sequence<ref name="SPRITE">Nadzirin, N.; Gardiner, E.; Willett, P.; Artymiuk, P. J.; Firdaus-Raih, M. 2012. SPRITE and ASSAM: web servers for side chain 3D-motif searching in protein structures. Nucleic Acids Res., 40(Web Server Issue), W380-6.</ref> Each chain is 266 residues long, and the entire complex has a molecular weight of approximately 58.5 kDa.<ref name="Sequence" />. |
[[Image:4Q7QABSequence.png|400px|left|thumb| Primary Sequences of the A and B chains of 4Q7Q.<ref name="SPRITE" />.]] | [[Image:4Q7QABSequence.png|400px|left|thumb| Primary Sequences of the A and B chains of 4Q7Q.<ref name="SPRITE" />.]] | ||
| Line 35: | Line 35: | ||
These enzymes also share similar secondary structures. Segments of alpha-helixes and beta-sheet strands appear and remain nearly entirely conserved throughout esterase analysis. A few conserved coils appear, but these sections do not appear as often as the other two secondary structures. | These enzymes also share similar secondary structures. Segments of alpha-helixes and beta-sheet strands appear and remain nearly entirely conserved throughout esterase analysis. A few conserved coils appear, but these sections do not appear as often as the other two secondary structures. | ||
| - | [[Image:4Q7QSecondaryEsterases.png|300px|left|thumb| Conserved secondary structures of note between 4Q7Q and Esterases. <ref name="DALI" />.]] | + | [[Image:4Q7QSecondaryEsterases.png|300px|left|thumb| Conserved secondary structures of note between 4Q7Q and Esterases.<ref name="DALI" />.]] |
Similar conserved sequences could be found between 4Q7Q and lipases. The GDSI, GxND, and DGxH sequences can be seen from lipases like 7BXD.<ref name="DALI" /.><ref name="7BXD">7BXD. Protein Database, 2021. https://www.rcsb.org/structure/7BXD</ref> The same secondary structure segments can also be located in the lipases analyzed. | Similar conserved sequences could be found between 4Q7Q and lipases. The GDSI, GxND, and DGxH sequences can be seen from lipases like 7BXD.<ref name="DALI" /.><ref name="7BXD">7BXD. Protein Database, 2021. https://www.rcsb.org/structure/7BXD</ref> The same secondary structure segments can also be located in the lipases analyzed. | ||
| Line 41: | Line 41: | ||
=== Structural Analysis === | === Structural Analysis === | ||
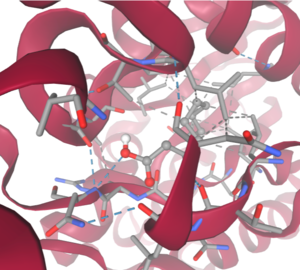
| - | Right-hand SPRITE analysis revealed 4Q7Q exhibited residues like those seen from enzymes operating with acetyl-like substrates. Specifically, residues Ser. 30, Gly. 69, Asn. 97, Asp. 251, and His. 254 on the A and B chains of 4Q7Q line up with similarly positioned residues on esterases like Platelet-Activating Factor Acetylhyd (PAFA), which exhibited an RMSD of 0.25 angstroms when compared to 4Q7Q. | + | Right-hand SPRITE analysis revealed 4Q7Q exhibited residues like those seen from enzymes operating with acetyl-like substrates.<ref name="SPRITE /> Specifically, residues Ser. 30, Gly. 69, Asn. 97, Asp. 251, and His. 254 on the A and B chains of 4Q7Q line up with similarly positioned residues on esterases like Platelet-Activating Factor Acetylhyd (PAFA), which exhibited an RMSD of 0.25 angstroms when compared to 4Q7Q.<ref name="SPRITE" /> |
| - | + | [[Image:4Q7QPAFASPRITE.png|300px|right|thumb|SPRITE-based alignment between motifs from 4Q7Q and PAFA.<ref name="SPRITE" />.]] | |
| - | + | Other proteins with similar motifs of note are Thioesterase I and Rhamnogalacturonan Acetylesterase, with RMSD values of 0.46 and 0.61, respectively.<ref name="SPRITE" /><ref name="Molgaard" /> These alignments focus on the same active site as PAFA did, suggesting the acetyl-like substrates 4Q7Q focuses on are similar to esters. | |
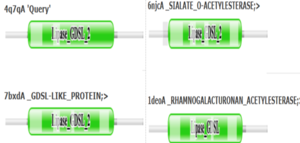
| - | 4Q7Q’s | + | PFAM graphics from DALI revealed significant structural equivalence between 4Q7Q, a lipase-like protein, Rhamnogalacturonan Acetylesterase, and Sialate O-acetylesterase.<ref name="DALI" /> |
| + | |||
| + | [[Image:4Q7QpfamLipases.png|300px|left|thumb|Pfam similarities between 4Q7Q and other enzymes.<ref name="DALI" />.]] | ||
| + | |||
| + | 4Q7Q’s superfamily also supports its SPRITE-derived hypothetical functionality. Rhamnogalacturonan Acetylesterase—the enzyme with one of the best SPRITE-based alignment relative to 4Q7Q—is a member of this family.<ref name="Molaard" /><ref name="SPRITE" /> Proteins in this family are also known for containing a “unique hydrogen bond network that [stabilizes]” the active site.<ref name="SPRITE" /> | ||
== Proposed Functionality == | == Proposed Functionality == | ||
| Line 53: | Line 57: | ||
=== Substrates and Docking Analysis === | === Substrates and Docking Analysis === | ||
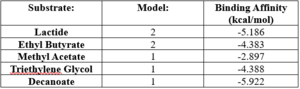
| - | SwissDock analysis showed a preference for larger molecules, specifically fatty acids. Lactide, Ethyl Butyrate, and Triethylene Glycol exhibited noticeably weak binding affinities to the theorized active site of 4Q7Q. These ligands may be ill-suited to act as substrates for 4Q7Q as they are remarkably polar, and lipids—one of the potential categories of substrates for 4Q7Q—are mostly non-polar. | + | SwissDock analysis showed a preference for larger molecules, specifically fatty acids. Lactide, Ethyl Butyrate, and Triethylene Glycol exhibited noticeably weak binding affinities to the theorized active site of 4Q7Q. <ref name="SwissDockOne">Bugnon M, Röhrig UF, Goullieux M, Perez MAS, Daina A, Michielin O, Zoete V. SwissDock 2024: major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52 (W1), W324-W332. DOI: 10.1093/nar/gkae300.</ref><ref name="SwissDockTwo"> |
| + | Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39 (Web Server issue), W270-W277. DOI: 10.1093/nar/gkr366</ref><ref name="SwissDockThree"> | ||
| + | Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model., 2021, 61 (8), 3891–3898, DOI: 10.1021/acs.jcim.1c00203</ref><ref name="SwissDockFour">Trott O, Olson AJ. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem., 2010, 31 (2), 455–461, DOI: 10.1002/jcc.21334</ref> These ligands may be ill-suited to act as substrates for 4Q7Q as they are remarkably polar, and lipids—one of the potential categories of substrates for 4Q7Q—are mostly non-polar.<ref name="SwissDockOne" /><ref name="SwissDockTwo" /><ref name="SwissDockThree" /><ref name="SwissDockFour /> | ||
| + | |||
| + | [[Image:DecanoateDocking.png|300px|right|thumb|SwissDock-based analysis of the intermolecular interactions between Decanoate and 4Q7Q's proposed active site <ref name="SwissDockOne" /><ref name="SwissDockTwo" /><ref name="SwissDockThree" /><ref name="SwissDockFour />]] | ||
Despite this, these ligands show noticeable hydrophobic interactions with the active site. This implies 4Q7Q uses hydrophobic regions to help guide substrates into the right orientation for enzymatic processes. This also further supports the possibility that 4Q7Q primarily operates with hydrophobic lipid-based substrates. This also explains why Methyl Acetate exhibited a relatively weaker affinity for 4Q7Q, as its smaller structure prevented hydrophobic interactions. | Despite this, these ligands show noticeable hydrophobic interactions with the active site. This implies 4Q7Q uses hydrophobic regions to help guide substrates into the right orientation for enzymatic processes. This also further supports the possibility that 4Q7Q primarily operates with hydrophobic lipid-based substrates. This also explains why Methyl Acetate exhibited a relatively weaker affinity for 4Q7Q, as its smaller structure prevented hydrophobic interactions. | ||
| + | |||
| + | [[Image:4Q7QDockingEnergies.png|300px|left|thumb|SwissDock-based analysis of the intermolecular interactions between Decanoate and 4Q7Q's proposed active site <ref name="SwissDockOne" /><ref name="SwissDockTwo" /><ref name="SwissDockThree" /><ref name="SwissDockFour />]] | ||
=== Hypothetical Function === | === Hypothetical Function === | ||
| + | |||
| + | Taking all of the above evidence into consideration, we currently believe 4Q7Q is an enzyme responsible for the hydrolysis of lipid esters and/or fatty acids via a serine protease active site. This may suggest 4Q7Q plays an important role in providing energy to the body in a method similar to the beta-oxidation of fatty acids <ref name="Textbook">Miesfeld, R. L.; McEvoy, M. M. Biochemistry, 2nd ed.; W. W. Norton & Company, 2021</ref> Other studies suggest that the hydrolysis of fatty acids could be involved in fermentation-related processes or even the degradation of aryl lipid esters.<ref name="sausage">Xia, L.; Qian, M.; Cheng, F.; Wang, Y.; Han, J.; Xu, Y.; Zhang, K.; Tian, J.; Jin, Y. The effect of lactic acid bacteria on lipid metabolism and flavor of fermented sausages. Food Biosci., 2023, 56, 103172.</ref><ref name="GDSL" /> | ||
| + | |||
| + | We also believe 4Q7Q undergoes several significant structural changes during enzymatic activities. Analysis into other members of its family reveal mechanisms wherein serine and histidine residues shift during substrate binding. <ref name="GDSL" /> | ||
=== Experimental Data === | === Experimental Data === | ||
Revision as of 21:38, 25 April 2025
4Q7Q Structure and Proposed Functionality
(NOTE TO ALL EDITORS: This page is part of a final project for a biochemistry lab at Elizabethtown College. Please do not edit this.)
4Q7Q is a homodimeric protein complex that originates from the bacterial species Chitinophaga Pinensis and has a mass of 58.5 kDa. It is a member of the SGNH Hydrolase Superfamily with structural and sequential similarities to esterases and lipases. Current evidence suggests it causes the hydrolysis of esters and/or acetyl groups on lipids/lipid-like molecules via a serine protease-like active site.
| |||||||||||
References
- ↑ 1.0 1.1 4Q7Q. Protein Database, 2014. https://www.rcsb.org/structure/4Q7Q
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Nadzirin, N.; Gardiner, E.; Willett, P.; Artymiuk, P. J.; Firdaus-Raih, M. 2012. SPRITE and ASSAM: web servers for side chain 3D-motif searching in protein structures. Nucleic Acids Res., 40(Web Server Issue), W380-6.
- ↑ 3.0 3.1 Rio, T. G. D.; et al. Complete genome sequence of Chitinophaga pinensis type strain (UQM 2034). Stand. Genomic. Sci., 2010, 2(1), 87-95. https://pmc.ncbi.nlm.nih.gov/articles/PMC3035255/
- ↑ 4.0 4.1 4.2 SGNH hydrolase superfamily. InterPro, 2017. https://www.ebi.ac.uk/interpro/entry/InterPro/IPR036514/
- ↑ 5.0 5.1 5.2 Rio, T. G. D.; et al. Complete genome sequence of Chitinophaga pinensis type strain (UQM 2034). Stand. Genomic. Sci., 2010, 2(1), 87-95. https://pmc.ncbi.nlm.nih.gov/articles/PMC3035255/
- ↑ 6.0 6.1 6.2 Molgaard, A.; Kauppinen, S.; Larsen, S. Rhamnogalacturonan acetylesterase elucidates the structure and function of a new family of hydrolases. Struct., 2000, 8(4), 373-383. https://www.sciencedirect.com/science/article/pii/S0969212600001180?via%3Dihub
- ↑ UCSF Chimera--a visualization system for exploratory research and analysis. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. J Comput Chem. 2004 Oct;25(13):1605-12.
- ↑ Akoh, C. C.; Lee, G.; Liaw, Y.; Huang, T.; Shaw, J. GDSL family of serine esterases/lipases. Prog. Lipid Res., 2004, 43(6), 534-552. https://pubmed.ncbi.nlm.nih.gov/15522763/
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Holm L, Laiho A, Toronen P, Salgado M (2023) DALI shines a light on remote homologs: one hundred discoveries. Protein Science 23, e4519
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMolaard - ↑ 11.0 11.1 11.2 11.3 Bugnon M, Röhrig UF, Goullieux M, Perez MAS, Daina A, Michielin O, Zoete V. SwissDock 2024: major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52 (W1), W324-W332. DOI: 10.1093/nar/gkae300.
- ↑ 12.0 12.1 12.2 12.3 Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39 (Web Server issue), W270-W277. DOI: 10.1093/nar/gkr366
- ↑ 13.0 13.1 13.2 13.3 Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model., 2021, 61 (8), 3891–3898, DOI: 10.1021/acs.jcim.1c00203
- ↑ Trott O, Olson AJ. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem., 2010, 31 (2), 455–461, DOI: 10.1002/jcc.21334
- ↑ Miesfeld, R. L.; McEvoy, M. M. Biochemistry, 2nd ed.; W. W. Norton & Company, 2021
- ↑ Xia, L.; Qian, M.; Cheng, F.; Wang, Y.; Han, J.; Xu, Y.; Zhang, K.; Tian, J.; Jin, Y. The effect of lactic acid bacteria on lipid metabolism and flavor of fermented sausages. Food Biosci., 2023, 56, 103172.