User:Hayden Vissing/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
===Overview of Active Site=== | ===Overview of Active Site=== | ||
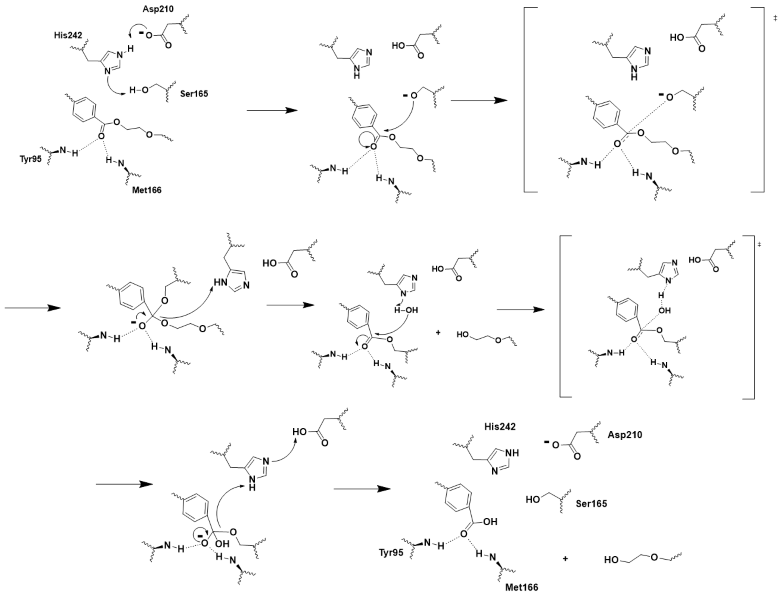
LCC is a serine hydrolase. The mechanism uses a <scene name='10/1076051/Wt_cat_triad/3'>catalytic triad</scene>: S165 (which was made into an alanine for crystallization purposes), D210, and H242. S165 is the nucleophile attacking the electrophilic carbonyl carbon with the <scene name='10/1075218/Oxyanion_hole/6'>oxyanion hole</scene> to stabilize the translation state<ref>Han, X., Liu, W., Huang, J. W., et al. (2017). Structural insight into catalytic mechanism of PET hydrolase. Nature Communications, 8, 2106. https://doi.org/10.1038/s41467-017-02255-z.Heredia-Guerrero, J. A., Heredia, A., García-Segura, R., & Benítez, J. J. (2009). Synthesis and characterization of a plant cutin mimetic polymer. Polymer, 50(24), 5633–5637. [https://doi.org/10.1016/j.polymer.2009.10.018 DOI: 10.1016/j.polymer.2009.10.018]</ref>. The model substrate for the reaction was <scene name='10/1075218/Substrate/4'>2-HE(MHET)3</scene><ref name="substrate">A binding model of the substrate 2-HE(MHET)3 in wild-type LLC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference “3”. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.</ref>. These key residues of the catalytic triad and oxyanion hole along with the 2-HE(MHET)3 substrate help to make up the <scene name='10/1076051/Wt_active_site/2'>active site</scene><ref name="main" />. | LCC is a serine hydrolase. The mechanism uses a <scene name='10/1076051/Wt_cat_triad/3'>catalytic triad</scene>: S165 (which was made into an alanine for crystallization purposes), D210, and H242. S165 is the nucleophile attacking the electrophilic carbonyl carbon with the <scene name='10/1075218/Oxyanion_hole/6'>oxyanion hole</scene> to stabilize the translation state<ref>Han, X., Liu, W., Huang, J. W., et al. (2017). Structural insight into catalytic mechanism of PET hydrolase. Nature Communications, 8, 2106. https://doi.org/10.1038/s41467-017-02255-z.Heredia-Guerrero, J. A., Heredia, A., García-Segura, R., & Benítez, J. J. (2009). Synthesis and characterization of a plant cutin mimetic polymer. Polymer, 50(24), 5633–5637. [https://doi.org/10.1016/j.polymer.2009.10.018 DOI: 10.1016/j.polymer.2009.10.018]</ref>. The model substrate for the reaction was <scene name='10/1075218/Substrate/4'>2-HE(MHET)3</scene><ref name="substrate">A binding model of the substrate 2-HE(MHET)3 in wild-type LLC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference “3”. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.</ref>. These key residues of the catalytic triad and oxyanion hole along with the 2-HE(MHET)3 substrate help to make up the <scene name='10/1076051/Wt_active_site/2'>active site</scene><ref name="main" />. | ||
| - | Using this knowledge of the chemistry of the 2-HE(MHET)3 substrate molecule, residues of the catalytic triad, and residues of the oxyanion hole, we were able to create an extensive push-mechanism for the hydrolysis of PET. The mechanism is drawn and shown in Figure 3. | + | Using this knowledge of the chemistry of the 2-HE(MHET)3 substrate molecule, residues of the catalytic triad, and residues of the oxyanion hole, we were able to create an extensive push-mechanism for the hydrolysis of PET. H242 and D210 played critical roles in maintaining the general acid-base catalysis, as well as the catalytic S165 serving as the needed nucleophile. Y95 and M166 of the oxyanion hole stabilize each of the two transition states and enhance the nuclopehilic attacks. The mechanism is drawn and shown in Figure 3. |
[[Image:Push_mechanism.png|800px|center|thumb|Figure 3 - Push-mechanism for the cutinase reaction]] | [[Image:Push_mechanism.png|800px|center|thumb|Figure 3 - Push-mechanism for the cutinase reaction]] | ||
Revision as of 00:11, 28 April 2025
The Future of Recycling: PET Hydrolase Enzyme with Improved Efficiency and Stability
| |||||||||||
References
- ↑ Hiraga, K., Taniguchi, I., Yoshida, S., Kimura, Y., & Oda, K. (2019). Biodegradation of waste PET: A sustainable solution for dealing with plastic pollution. EMBO Reports, 20(11), e49365. https://doi.org/10.15252/embr.201949365. [Published correction appears in EMBO Reports, 21(2), e49826. DOI: 10.15252/embr.201949826
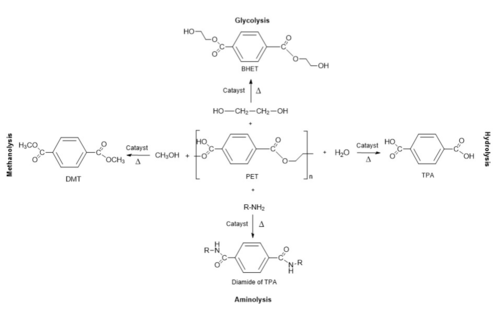
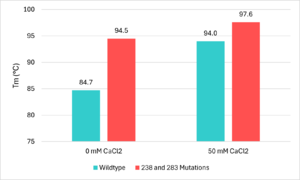
- ↑ 2.0 2.1 2.2 2.3 Jayasekara, S. K., Joni, H. D., Jayantha, B., Dissanayake, L., Mandrell, C., Sinharage, M. M. S., Molitor, R., Jayasekara, T., Sivakumar, P., & Jayakody, L. N. (2023). Trends in in-silico guided engineering of efficient polyethylene terephthalate (PET) hydrolyzing enzymes to enable bio-recycling and upcycling of PET. Computational and structural biotechnology journal, 21, 3513–3521. DOI: 10.1016/j.csbj.2023.06.004
- ↑ 3.0 3.1 Babaei, M., Jalilian, M., & Shahbaz, K. (2024). Chemical recycling of Polyethylene terephthalate: A mini-review. Journal of Environmental Chemical Engineering, 12(3), 112507. DOI: 10.1016/j.jece.2024.112507
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ Joo S, Cho IJ, Seo H, Son HF, Sagong HY, Shin TJ, Choi SY, Lee SY, Kim KJ. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat Commun. 2018 Jan 26;9(1):382. doi: 10.1038/s41467-018-02881-1. PMID:29374183 doi:http://dx.doi.org/10.1038/s41467-018-02881-1
- ↑ Sulaiman S, Yamato S, Kanaya E, Kim JJ, Koga Y, Takano K, Kanaya S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl Environ Microbiol. 2012 Mar;78(5):1556-62. doi: 10.1128/AEM.06725-11. Epub, 2011 Dec 22. PMID:22194294 doi:http://dx.doi.org/10.1128/AEM.06725-11
- ↑ Han, X., Liu, W., Huang, J. W., et al. (2017). Structural insight into catalytic mechanism of PET hydrolase. Nature Communications, 8, 2106. https://doi.org/10.1038/s41467-017-02255-z.Heredia-Guerrero, J. A., Heredia, A., García-Segura, R., & Benítez, J. J. (2009). Synthesis and characterization of a plant cutin mimetic polymer. Polymer, 50(24), 5633–5637. DOI: 10.1016/j.polymer.2009.10.018
- ↑ A binding model of the substrate 2-HE(MHET)3 in wild-type LLC (4eb0.pdb) was constructed and refined to mimic the 3D structure illustrated in Figure 2 of reference “3”. The software Maestro (Schrödinger, Inc; version 14.2.118) was used to construct the initial binding structure, followed by energy minimization in the context of the rigid protein that had previously been processed to add/refine all hydrogen atoms. The ligand model was then used without further modification to identify and illustrate the cited active-site residues.
Additional Literature and Resources
- Roth C, Wei R, Oeser T, Then J, Follner C, Zimmermann W, Strater N. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl Microbiol Biotechnol. 2014 Apr 13. PMID:24728714 doi:http://dx.doi.org/10.1007/s00253-014-5672-0
Student Contributors
- David Bogle
- Justin Chavez
- Hayden Vissing