We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1849
From Proteopedia
(Difference between revisions)
| Line 47: | Line 47: | ||
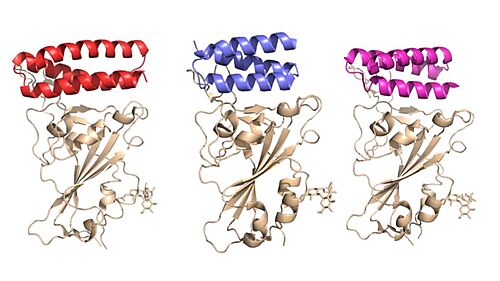
These minibinders were designed to form stronger connections to the spike protein RBD than ACE2<ref name="Longxing">PMID:32907861</ref>. This section highlights some important residue connections with the RBD that give the minibinders higher affinities for the spike protein than ACE2. | These minibinders were designed to form stronger connections to the spike protein RBD than ACE2<ref name="Longxing">PMID:32907861</ref>. This section highlights some important residue connections with the RBD that give the minibinders higher affinities for the spike protein than ACE2. | ||
| - | ACE2 binds to the spike protein RBD using these residues. The minibinders are smaller than ACE2, but still form strong connections to the spike protein. This means the minibinders interact with the RBD residues more efficiently than ACE2 giving them a higher affinity for the spike protein <ref name="Longxing">PMID:32907861</ref>. AHB2 was designed to mimic ACE2 while making better use of the RBD residues giving it a higher affinity for the spike protein with an IC<sub>50</sub> value of 15.5 nM<ref name="Longxing">PMID:32907861</ref> (AHB2 and RBD binding residues). LCB1 was designed from scratch to bind the most efficiently to the RBD residues giving it the highest affinity with the lowest IC<sub>50</sub> value of 23.5 pM<ref name="Longxing">PMID:32907861</ref> (LCB1 and RBD binding residues). LCB3 was designed after LCB1 with the goal of making the minibinder even more efficient, however it ended up having a lower affinity than LCB1 with a higher IC<sub>50</sub> value of 40.1 pM<ref name="Longxing">PMID:32907861</ref> (LCB3 and RBD binding residues). | + | ACE2 binds to the spike protein RBD using <scene name='10/1075250/Ace2_allbinding/2'>these residues</scene>. The minibinders are smaller than ACE2, but still form strong connections to the spike protein. This means the minibinders interact with the RBD residues more efficiently than ACE2 giving them a higher affinity for the spike protein <ref name="Longxing">PMID:32907861</ref>. AHB2 was designed to mimic ACE2 while making better use of the RBD residues giving it a higher affinity for the spike protein with an IC<sub>50</sub> value of 15.5 nM<ref name="Longxing">PMID:32907861</ref> (AHB2 and RBD binding residues). LCB1 was designed from scratch to bind the most efficiently to the RBD residues giving it the highest affinity with the lowest IC<sub>50</sub> value of 23.5 pM<ref name="Longxing">PMID:32907861</ref> (<scene name='10/1075250/Lcb1_binding_residues/5'>LCB1 and RBD binding residues</scene>). LCB3 was designed after LCB1 with the goal of making the minibinder even more efficient, however it ended up having a lower affinity than LCB1 with a higher IC<sub>50</sub> value of 40.1 pM<ref name="Longxing">PMID:32907861</ref> (LCB3 and RBD binding residues). |
The Glutamine-493 residue on the spike protein is an important residue in showing the differences in strength between ACE2 and the minibinders <ref name="Longxing">PMID:32907861</ref>. ACE2 does not make use of this residue when binding to the RBD. The nearest residues of ACE2, Gln35 and Lys31 do not form any interaction on Gln493 of the spike protein. The AHB2 minibinder, instead forms a Hydrogen bond on the Gln493 residue of the spike protein with its Glu41, increasing affinity to the spike protein. LCB1 forms two hydrogen bonds on Gln493 of the spike protein using two different residues, giving it the highest affinity based on the Gln493 residue. However, these interactions are not required. LCB1 forms no interactions with the Gln493 residue of the RDB previously mentioned, yet it still has a higher affinity to the RBD than AHB2 which forms a hydrogen bond with the Gln493 residue. | The Glutamine-493 residue on the spike protein is an important residue in showing the differences in strength between ACE2 and the minibinders <ref name="Longxing">PMID:32907861</ref>. ACE2 does not make use of this residue when binding to the RBD. The nearest residues of ACE2, Gln35 and Lys31 do not form any interaction on Gln493 of the spike protein. The AHB2 minibinder, instead forms a Hydrogen bond on the Gln493 residue of the spike protein with its Glu41, increasing affinity to the spike protein. LCB1 forms two hydrogen bonds on Gln493 of the spike protein using two different residues, giving it the highest affinity based on the Gln493 residue. However, these interactions are not required. LCB1 forms no interactions with the Gln493 residue of the RDB previously mentioned, yet it still has a higher affinity to the RBD than AHB2 which forms a hydrogen bond with the Gln493 residue. | ||
Revision as of 18:04, 28 April 2025
| This Sandbox is Reserved from March 18 through September 1, 2025 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson and Mark Macbeth at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1828 through Sandbox Reserved 1846. |
To get started:
More help: Help:Editing |
Contents |
SARS-COV2 Minibinders
| |||||||||||
Color Key
■ -> ACE2
■ -> Spike RBD
■ -> AHB2
■ -> LCB1
■ -> LCB3
See Also
COVID-19
- Coronavirus Disease 2019 (COVID-19)
- COVID-19 (Wikipedia)
Spike Protein
- SARS-CoV-2_protein_S
- Coronavirus Spike Protein (Wikipedia)
- Spike protein
- SARS-CoV-2_spike_protein_mutations
- SARS-CoV-2_protein_S_priming_by_furin
- SARS-CoV-2_spike_protein_fusion_transformation
ACE2
Minibinders
- Not found
Misc
- Saturation mutagenesis (Wikipedia)
Contributions
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 Cao L, Goreshnik I, Coventry B, Case JB, Miller L, Kozodoy L, Chen RE, Carter L, Walls AC, Park YJ, Strauch EM, Stewart L, Diamond MS, Veesler D, Baker D. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science. 2020 Oct 23;370(6515):426-431. PMID:32907861 doi:10.1126/science.abd9909
- ↑ 2.0 2.1 2.2 2.3 2.4 Case JB, Chen RE, Cao L, Ying B, Winkler ES, Johnson M, Goreshnik I, Pham MN, Shrihari S, Kafai NM, Bailey AL, Xie X, Shi PY, Ravichandran R, Carter L, Stewart L, Baker D, Diamond MS. Ultrapotent miniproteins targeting the SARS-CoV-2 receptor-binding domain protect against infection and disease. Cell Host Microbe. 2021 Jul 14;29(7):1151-1161.e5. PMID:34192518 doi:10.1016/j.chom.2021.06.008
- ↑ Sang P, Chen YQ, Liu MT, Wang YT, Yue T, Li Y, Yin YR, Yang LQ. Electrostatic Interactions Are the Primary Determinant of the Binding Affinity of SARS-CoV-2 Spike RBD to ACE2: A Computational Case Study of Omicron Variants. Int J Mol Sci. 2022 Nov 26;23(23):14796. PMID:36499120 doi:10.3390/ijms232314796
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020 Sep;41(9):1141-1149. doi: 10.1038/s41401-020-0485-4., Epub 2020 Aug 3. PMID:32747721 doi:http://dx.doi.org/10.1038/s41401-020-0485-4
- ↑ Cao L, Goreshnik I, Coventry B, Case JB, Miller L, Kozodoy L, Chen RE, Carter L, Walls AC, Park YJ, Strauch EM, Stewart L, Diamond MS, Veesler D, Baker D. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science. 2020 Oct 23;370(6515):426-431. PMID:32907861 doi:10.1126/science.abd9909
- ↑ 6.0 6.1 6.2 Zhang J, Xiao T, Cai Y, Chen B. Structure of SARS-CoV-2 spike protein. Curr Opin Virol. 2021 Oct;50:173-182. PMID:34534731 doi:10.1016/j.coviro.2021.08.010
- ↑ 7.0 7.1 Yuan Y, Cao D, Zhang Y, Ma J, Qi J, Wang Q, Lu G, Wu Y, Yan J, Shi Y, Zhang X, Gao GF. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017 Apr 10;8:15092. doi: 10.1038/ncomms15092. PMID:28393837 doi:http://dx.doi.org/10.1038/ncomms15092
- ↑ 8.0 8.1 Kuba K, Yamaguchi T, Penninger JM. Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19. Front Immunol. 2021 Dec 22;12:732690. PMID:35003058 doi:10.3389/fimmu.2021.732690
- ↑ Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005 Aug;11(8):875-9. PMID:16007097 doi:10.1038/nm1267
- ↑ 10.0 10.1 Valetti F, Gilardi G. Improvement of biocatalysts for industrial and environmental purposes by saturation mutagenesis. Biomolecules. 2013 Oct 8;3(4):778-811. PMID:24970191 doi:10.3390/biom3040778