We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Matthew Chien/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
[[Image:Ijms-22-05125-g008.jpg|thumb|400px|C1q CLR active site residues Lys58 and Lys61]] | [[Image:Ijms-22-05125-g008.jpg|thumb|400px|C1q CLR active site residues Lys58 and Lys61]] | ||
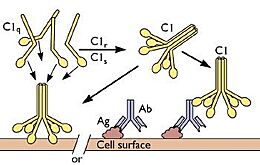
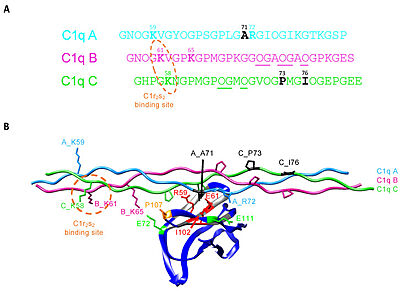
The collagen-like region (CLR) is the location of a binding site associated with a variety of non-complement related interactions, such as the binding of phagocytes to C1q as mentioned above. However, one of the most important functions of the CLR of C1q is the activation through protease of the remainder of C1; C1r and C1s. Two equivalences of C1r and C1s are bonded C1s - C1r - C1r - C1s to comprise a tetramer that, when cleaved after the bonding of C1q to an epitope, activates the classical pathway of complement activity, promoting inflammation and phagocytosis. The active site on the CLR of C1q is proposed to be lysine residues located at position 61 on the B chain and position 58 on the A chain. These basic residues create salt bridges with associated residues on C1r, generally a glutamic acid or aspartic acid. These residues are coordinated with the Ca2+ ion present in C1q through single carboxyl oxygens, which is available for mediating through an electrostatic bond with a basic residue. Through activation, the C1r - C1r bond is broken, which induces conformational changes in C1r that subsequently cleaves C1s. <ref>PMID:29311313</ref><ref>PMID:23922389</ref><ref>PMID:1939090</ref><ref>PMID:34066122</ref> | The collagen-like region (CLR) is the location of a binding site associated with a variety of non-complement related interactions, such as the binding of phagocytes to C1q as mentioned above. However, one of the most important functions of the CLR of C1q is the activation through protease of the remainder of C1; C1r and C1s. Two equivalences of C1r and C1s are bonded C1s - C1r - C1r - C1s to comprise a tetramer that, when cleaved after the bonding of C1q to an epitope, activates the classical pathway of complement activity, promoting inflammation and phagocytosis. The active site on the CLR of C1q is proposed to be lysine residues located at position 61 on the B chain and position 58 on the A chain. These basic residues create salt bridges with associated residues on C1r, generally a glutamic acid or aspartic acid. These residues are coordinated with the Ca2+ ion present in C1q through single carboxyl oxygens, which is available for mediating through an electrostatic bond with a basic residue. Through activation, the C1r - C1r bond is broken, which induces conformational changes in C1r that subsequently cleaves C1s. <ref>PMID:29311313</ref><ref>PMID:23922389</ref><ref>PMID:1939090</ref><ref>PMID:34066122</ref> | ||
| + | |||
| + | == Structural Highlights == | ||
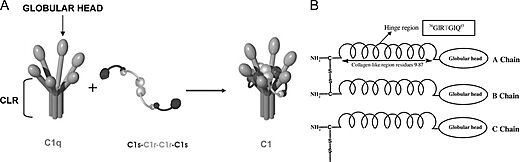
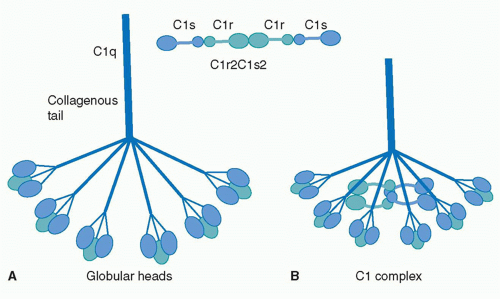
| + | C1q is a 460 kDa protein complex composed of six collagen-like stems, each linked to a globular head. This produces a bouquet-like structure shown below. Each collagen-like stem is comprised of three separate chains that form a triple helix structure, totaling 18 polypeptide chains of three different types; A, B, and C. Each chain has an N-terminus in at the CLR and a <scene name='10/1078778/C-termini/1'>C-terminus</scene> at the globular head. Disulfide bonds link the N-terminus ends of A and B chains, with these dimers being linked to the C chains noncovalently. [[Image:Schematic-representation-of-structural-organization-of-human-C1q-and-of-C1-assembly-A.jpeg|thumb|right|520px|N-terminus of C1q]] These form the triple helices that attach to the globular head, making the bouquet-like structure. The globular heads are comprised of three independently folding domains, making them heterotrimeric. The globular heads are very compact and held together by both electrostatic and nonpolar interactions. Each globular head contains a <scene name='10/1078778/Calcium_ion/1'>calcium ion</scene>, responsible for target recognition properties and electrostatic stability. At pH 7.4, the Calcium ion is lost from the globular head, which contributes to IgG binding site recognition. <ref>PMID:23650384</ref><ref>PMID:16245926</ref> | ||
| + | [[Image:Frame apngframe1.png]] | ||
== Disease == | == Disease == | ||
| Line 34: | Line 38: | ||
An overabundance of C1q has shown to correlate with the development of neurodegenerative diseases and loss of cognitive function. Higher age groups are seen to have larger levels of C1q, especially in their brain. This buildup mainly occurs because of poorer activity in synaptic clearing of C1q bound to neuronal RNA-binding proteins called neuronal ribonucleoprotein complexes, affecting protein homeostasis in the brain and decreasing cognitive function. C1q is involved in pruning synapses in developing brains by tagging the synapses for phagocytosis by microglia. Elevated concentrations of C1q in the synapses has been correlated with overactivity of microglia when provoked by brain injury or a series of strokes. Most cells in the body have complement inhibiting agents to regulate complement activity, whereas nerve cells lack these complement inhibitors. Astrocytes are known to secrete C1q when provoked by infections or damage to the central nervous system, and an overproduction of these can lead to over inflammation in the brain by the complement cascade and synapses loss, both fundamental components of Alzheimer's disease and various other neurodegenerative diseases. <ref>PMID:23946404</ref><ref>PMID:38942014</ref><ref>PMID:37033981</ref> | An overabundance of C1q has shown to correlate with the development of neurodegenerative diseases and loss of cognitive function. Higher age groups are seen to have larger levels of C1q, especially in their brain. This buildup mainly occurs because of poorer activity in synaptic clearing of C1q bound to neuronal RNA-binding proteins called neuronal ribonucleoprotein complexes, affecting protein homeostasis in the brain and decreasing cognitive function. C1q is involved in pruning synapses in developing brains by tagging the synapses for phagocytosis by microglia. Elevated concentrations of C1q in the synapses has been correlated with overactivity of microglia when provoked by brain injury or a series of strokes. Most cells in the body have complement inhibiting agents to regulate complement activity, whereas nerve cells lack these complement inhibitors. Astrocytes are known to secrete C1q when provoked by infections or damage to the central nervous system, and an overproduction of these can lead to over inflammation in the brain by the complement cascade and synapses loss, both fundamental components of Alzheimer's disease and various other neurodegenerative diseases. <ref>PMID:23946404</ref><ref>PMID:38942014</ref><ref>PMID:37033981</ref> | ||
| - | == Structural Highlights == | ||
| - | C1q is a 460 kDa protein complex composed of six collagen-like stems, each linked to a globular head. This produces a bouquet-like structure shown below. Each collagen-like stem is comprised of three separate chains that form a triple helix structure, totaling 18 polypeptide chains of three different types; A, B, and C. Each chain has an N-terminus in at the CLR and a <scene name='10/1078778/C-termini/1'>C-terminus</scene> at the globular head. Disulfide bonds link the N-terminus ends of A and B chains, with these dimers being linked to the C chains noncovalently. [[Image:Schematic-representation-of-structural-organization-of-human-C1q-and-of-C1-assembly-A.jpeg|thumb|right|520px|N-terminus of C1q]] These form the triple helices that attach to the globular head, making the bouquet-like structure. The globular heads are comprised of three independently folding domains, making them heterotrimeric. The globular heads are very compact and held together by both electrostatic and nonpolar interactions. Each globular head contains a <scene name='10/1078778/Calcium_ion/1'>calcium ion</scene>, responsible for target recognition properties and electrostatic stability. At pH 7.4, the Calcium ion is lost from the globular head, which contributes to IgG binding site recognition. <ref>PMID:23650384</ref><ref>PMID:16245926</ref> | ||
| - | [[Image:Frame apngframe1.png]] | ||
== Additional Resources == | == Additional Resources == | ||
*[https://www.youtube.com/watch?v=IUDSWPvfHgU Classical Pathway Activation] animation of C1q binding to IgG, initiating the classical pathway of the complement system. | *[https://www.youtube.com/watch?v=IUDSWPvfHgU Classical Pathway Activation] animation of C1q binding to IgG, initiating the classical pathway of the complement system. | ||
Revision as of 14:20, 29 April 2025
Complement Component 1q
| |||||||||||
References
- ↑ Reid KBM. Complement Component C1q: Historical Perspective of a Functionally Versatile, and Structurally Unusual, Serum Protein. Front Immunol. 2018 Apr 10;9:764. PMID:29692784 doi:10.3389/fimmu.2018.00764
- ↑ Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The complement system and innate immunity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27100/
- ↑ Kishore U, Ghai R, Greenhough TJ, Shrive AK, Bonifati DM, Gadjeva MG, Waters P, Kojouharova MS, Chakraborty T, Agrawal A. Structural and functional anatomy of the globular domain of complement protein C1q. Immunol Lett. 2004 Sep;95(2):113-28. PMID:15388251 doi:10.1016/j.imlet.2004.06.015
- ↑ Kaul M, Loos M. Dissection of C1q capability of interacting with IgG. Time-dependent formation of a tight and only partly reversible association. J Biol Chem. 1997 Dec 26;272(52):33234-44. PMID:9407113 doi:10.1074/jbc.272.52.33234
- ↑ Mortensen SA, Sander B, Jensen RK, Pedersen JS, Golas MM, Jensenius JC, Hansen AG, Thiel S, Andersen GR. Structure and activation of C1, the complex initiating the classical pathway of the complement cascade. Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):986-991. PMID:28104818 doi:10.1073/pnas.1616998114
- ↑ Sontheimer RD, Racila E, Racila DM. C1q: its functions within the innate and adaptive immune responses and its role in lupus autoimmunity. J Invest Dermatol. 2005 Jul;125(1):14-23. PMID:15982298 doi:10.1111/j.0022-202X.2005.23673.x
- ↑ Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010 Jan;20(1):34-50. PMID:20010915 doi:10.1038/cr.2009.139
- ↑ Marqués G, Antón LC, Barrio E, Sánchez A, Ruiz S, Gavilanes F, Vivanco F. Arginine residues of the globular regions of human C1q involved in the interaction with immunoglobulin G. J Biol Chem. 1993 May 15;268(14):10393-402 PMID:8486696
- ↑ Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988 Apr 21;332(6166):738-40. PMID:3258649 doi:10.1038/332738a0
- ↑ Almitairi JOM, Venkatraman Girija U, Furze CM, Simpson-Gray X, Badakshi F, Marshall JE, Schwaeble WJ, Mitchell DA, Moody PCE, Wallis R. Structure of the C1r-C1s interaction of the C1 complex of complement activation. Proc Natl Acad Sci U S A. 2018 Jan 8. pii: 1718709115. doi:, 10.1073/pnas.1718709115. PMID:29311313 doi:http://dx.doi.org/10.1073/pnas.1718709115
- ↑ Venkatraman Girija U, Gingras AR, Marshall JE, Panchal R, Sheikh MA, Gal P, Schwaeble WJ, Mitchell DA, Moody PC, Wallis R. Structural basis of the C1q/C1s interaction and its central role in assembly of the C1 complex of complement activation. Proc Natl Acad Sci U S A. 2013 Aug 20;110(34):13916-20. doi:, 10.1073/pnas.1311113110. Epub 2013 Aug 6. PMID:23922389 doi:10.1073/pnas.1311113110
- ↑ Guan EN, Burgess WH, Robinson SL, Goodman EB, McTigue KJ, Tenner AJ. Phagocytic cell molecules that bind the collagen-like region of C1q. Involvement in the C1q-mediated enhancement of phagocytosis. J Biol Chem. 1991 Oct 25;266(30):20345-55 PMID:1939090
- ↑ Fouët G, Bally I, Chouquet A, Reiser JB, Thielens NM, Gaboriaud C, Rossi V. Molecular Basis of Complement C1q Collagen-Like Region Interaction with the Immunoglobulin-Like Receptor LAIR-1. Int J Mol Sci. 2021 May 12;22(10):5125. PMID:34066122 doi:10.3390/ijms22105125
- ↑ Bally I, Ancelet S, Moriscot C, Gonnet F, Mantovani A, Daniel R, Schoehn G, Arlaud GJ, Thielens NM. Expression of recombinant human complement C1q allows identification of the C1r/C1s-binding sites. Proc Natl Acad Sci U S A. 2013 May 21;110(21):8650-5. PMID:23650384 doi:10.1073/pnas.1304894110
- ↑ Roumenina LT, Kantardjiev AA, Atanasov BP, Waters P, Gadjeva M, Reid KB, Mantovani A, Kishore U, Kojouharova MS. Role of Ca2+ in the electrostatic stability and the functional activity of the globular domain of human C1q. Biochemistry. 2005 Nov 1;44(43):14097-109. PMID:16245926 doi:10.1021/bi051186n
- ↑ Mahler M, van Schaarenburg RA, Trouw LA. Anti-C1q autoantibodies, novel tests, and clinical consequences. Front Immunol. 2013 May 14;4:117. PMID:23717311 doi:10.3389/fimmu.2013.00117
- ↑ Buck A, Christensen J, McCarty M. Hypocomplementemic urticarial vasculitis syndrome: a case report and literature review. J Clin Aesthet Dermatol. 2012 Jan;5(1):36-46 PMID:22328958
- ↑ Jayakanthan K, Gupta AN, Mathew J, Ravindran R, Mahasampth G, Danda D. Clinical utility of anti-C1q antibody in primary and secondary vasculitic conditions. Int J Health Sci (Qassim). 2017 Nov-Dec;11(5):3-6 PMID:29114186
- ↑ Stojan G, Petri M. Anti-C1q in systemic lupus erythematosus. Lupus. 2016 Jul;25(8):873-7. PMID:27252264 doi:10.1177/0961203316645205
- ↑ Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L, Kim L, Tsai HH, Huang EJ, Rowitch DH, Berns DS, Tenner AJ, Shamloo M, Barres BA. A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013 Aug 14;33(33):13460-74. PMID:23946404 doi:10.1523/JNEUROSCI.1333-13.2013
- ↑ Scott-Hewitt N, Mahoney M, Huang Y, Korte N, Yvanka de Soysa T, Wilton DK, Knorr E, Mastro K, Chang A, Zhang A, Melville D, Schenone M, Hartigan C, Stevens B. Microglial-derived C1q integrates into neuronal ribonucleoprotein complexes and impacts protein homeostasis in the aging brain. Cell. 2024 Aug 8;187(16):4193-4212.e24. PMID:38942014 doi:10.1016/j.cell.2024.05.058
- ↑ Zhang W, Chen Y, Pei H. C1q and central nervous system disorders. Front Immunol. 2023 Mar 23;14:1145649. PMID:37033981 doi:10.3389/fimmu.2023.1145649