We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Alexander Grayzel/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Structural highlights == | == Structural highlights == | ||
| - | Ferritin is a tetramer composed of 24 subunits (24-mer) forming a hollow spherical shell, with a total molecular weight of approximately 478 kDa and a diameter of 8.66 nm. These subunits exist in two primary forms in humans: heavy (H, 21 kDa) and light (L, 19 kDa) chains. These two chains co-assemble in various proportions (H:L) to form the iron-storage complex. The ratio of H:L is greater in tissues in which the activity of iron oxidation is at a high level and iron needs to be detoxified, for example the heart or brain. The make-up of the subunits in the shell does not affect the iron/oxy mineral composition in the core. What’s interesting is that two identical ferritin proteins, meaning proteins with the same H:L ratio, will likely have different iron cores. Additionally, the H:L ratio will have some effect on the geometry of the crystalline structure as their properties are different. | + | Ferritin is a tetramer composed of 24 <scene name='10/1078819/Single_chain_of_ferritin/1'>subunits</scene> (24-mer) forming a hollow spherical shell, with a total molecular weight of approximately 478 kDa and a diameter of 8.66 nm. These subunits exist in two primary forms in humans: heavy (H, 21 kDa) and light (L, 19 kDa) chains. These two chains co-assemble in various proportions (H:L) to form the iron-storage complex. The ratio of H:L is greater in tissues in which the activity of iron oxidation is at a high level and iron needs to be detoxified, for example the heart or brain. The make-up of the subunits in the shell does not affect the iron/oxy mineral composition in the core. What’s interesting is that two identical ferritin proteins, meaning proteins with the same H:L ratio, will likely have different iron cores. Additionally, the H:L ratio will have some effect on the geometry of the crystalline structure as their properties are different. |
| - | Each individual subunit of the 24-mer consists of five -helices and no -sheets, forming a couple of four-helix bundle (A-B and C-D) | + | Each individual subunit of the 24-mer consists of five -helices and no -sheets, forming a couple of four-helix bundle (A-B and C-D) connected by loops, with a short C-terminal helix (A) providing protein stabilization. The H-chain posses ferroxidase activity, while the L-chain supports iron nucleation and mineralization. Subunits share about 55% sequence identity. Iron channels on the ferritin surface are lined with polar side chains primarily of glutamate, which makes a hydrophilic channel allowing iron ions into the core. Additionally, the negative charge on glutamate acts as a good binding site for iron ions. |
<scene name='10/1078819/ConSurf-DB_Analysis_of_3KX9/1'>ConSurf analysis</scene> of chain A to see evolutionary, structural, and organismal significance. | <scene name='10/1078819/ConSurf-DB_Analysis_of_3KX9/1'>ConSurf analysis</scene> of chain A to see evolutionary, structural, and organismal significance. | ||
| Line 14: | Line 14: | ||
== Function == | == Function == | ||
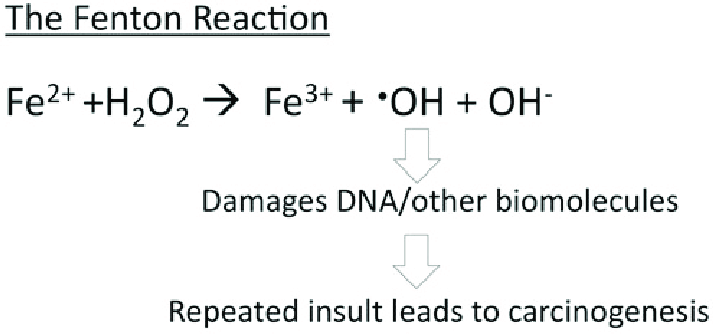
Ferritin stores iron in a safe, bioavailable form. By sequestering Fe³⁺ in a mineralized core, it prevents free iron from catalyzing harmful oxidative reactions. In addition to iron storage, ferritin contributes to intracellular iron delivery, especially during high-demand situations such as rapid growth repair. Its capacity to hold more iron than transferring makes it vital for systemic iron regulation. | Ferritin stores iron in a safe, bioavailable form. By sequestering Fe³⁺ in a mineralized core, it prevents free iron from catalyzing harmful oxidative reactions. In addition to iron storage, ferritin contributes to intracellular iron delivery, especially during high-demand situations such as rapid growth repair. Its capacity to hold more iron than transferring makes it vital for systemic iron regulation. | ||
| - | |||
| - | <scene name='10/1078819/Single_chain_of_ferritin/1'>Single chain</scene> | ||
<scene name='10/1078819/Ferritin_with_iron/1'>Ferritin with iron ion</scene> | <scene name='10/1078819/Ferritin_with_iron/1'>Ferritin with iron ion</scene> | ||
Revision as of 21:11, 30 April 2025
Ferritin
| |||||||||||
References
Chiou, Brian, and James R Connor. “Emerging and Dynamic Biomedical Uses of Ferritin.” Pharmaceuticals (Basel, Switzerland) vol. 11,4 124. 13 Nov. 2018, doi:10.3390/ph11040124