We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Alexander Grayzel/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
Ferritin is a tetramer composed of 24 <scene name='10/1078819/Single_chain_of_ferritin/1'>subunits</scene> (24-mer) forming a hollow spherical shell, with a total molecular weight of approximately 478 kDa and a diameter of 8.66 nm. <ref name="srivastava">Srivastava, A.K., Reutovich, A.A., Hunter, N.J. et al. Ferritin microheterogeneity, subunit composition, functional, and physiological implications. Sci Rep 13, 19862 (2023). https://doi.org/10.1038/s41598-023-46880-9</ref> These subunits exist in two primary forms in humans: heavy (H, 21 kDa) and light (L, 19 kDa) chains.<ref name="srivastava" /> These two chains co-assemble in various proportions (H:L) to form the iron-storage complex. The ratio of H:L is greater in tissues in which the activity of iron oxidation is at a high level and iron needs to be detoxified, for example the heart or brain. The make-up of the subunits in the shell does not affect the iron/oxy mineral composition in the core. What’s interesting is that two identical ferritin proteins, meaning proteins with the same H:L ratio, will likely have different iron cores. Additionally, the H:L ratio will have some effect on the geometry of the crystalline structure as their properties are different. | Ferritin is a tetramer composed of 24 <scene name='10/1078819/Single_chain_of_ferritin/1'>subunits</scene> (24-mer) forming a hollow spherical shell, with a total molecular weight of approximately 478 kDa and a diameter of 8.66 nm. <ref name="srivastava">Srivastava, A.K., Reutovich, A.A., Hunter, N.J. et al. Ferritin microheterogeneity, subunit composition, functional, and physiological implications. Sci Rep 13, 19862 (2023). https://doi.org/10.1038/s41598-023-46880-9</ref> These subunits exist in two primary forms in humans: heavy (H, 21 kDa) and light (L, 19 kDa) chains.<ref name="srivastava" /> These two chains co-assemble in various proportions (H:L) to form the iron-storage complex. The ratio of H:L is greater in tissues in which the activity of iron oxidation is at a high level and iron needs to be detoxified, for example the heart or brain. The make-up of the subunits in the shell does not affect the iron/oxy mineral composition in the core. What’s interesting is that two identical ferritin proteins, meaning proteins with the same H:L ratio, will likely have different iron cores. Additionally, the H:L ratio will have some effect on the geometry of the crystalline structure as their properties are different. | ||
| - | Each individual subunit of the 24-mer consists of five alpha-helices and no beta-sheets, forming a couple of four-helix bundle (A-B and C-D) connected by loops, with a short C-terminal helix (A) providing protein stabilization.<ref name="Levi">Levi, S., & Rovida, E. (2015). Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiology of disease, 81, 134–143. https://doi.org/10.1016/j.nbd.2015.02.007</ref> The H-chain posses ferroxidase activity, while the L-chain supports iron nucleation and mineralization. Subunits share about 55% sequence identity.<ref name="Levi" /> Iron channels on the ferritin surface are lined with polar side chains primarily of glutamate, which makes a hydrophilic channel allowing iron ions into the core. Additionally, the negative charge on glutamate acts as a good binding site for iron ions. | + | Each individual subunit of the 24-mer consists of five alpha-helices and no beta-sheets, forming a couple of four-helix bundle (A-B and C-D) connected by loops, with a short C-terminal helix (A) providing protein stabilization.<ref name="Levi">Levi, S., & Rovida, E. (2015). Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiology of disease, 81, 134–143. https://doi.org/10.1016/j.nbd.2015.02.007</ref> The H-chain posses <scene name='10/1078819/Ferritin_with_iron/1'>ferroxidase</scene> activity, while the L-chain supports iron nucleation and mineralization. Subunits share about 55% sequence identity.<ref name="Levi" /> Iron channels on the ferritin surface are lined with polar side chains primarily of glutamate, which makes a hydrophilic channel allowing iron ions into the core. Additionally, the negative charge on glutamate acts as a good binding site for iron ions. |
== Function == | == Function == | ||
Ferritin stores iron in a safe, bioavailable form. By sequestering Fe³⁺ in a mineralized core, it prevents free iron from catalyzing harmful oxidative reactions. In addition to iron storage, ferritin contributes to intracellular iron delivery, especially during high-demand situations such as rapid growth repair. Its capacity to hold more iron than transferring makes it vital for systemic iron regulation. | Ferritin stores iron in a safe, bioavailable form. By sequestering Fe³⁺ in a mineralized core, it prevents free iron from catalyzing harmful oxidative reactions. In addition to iron storage, ferritin contributes to intracellular iron delivery, especially during high-demand situations such as rapid growth repair. Its capacity to hold more iron than transferring makes it vital for systemic iron regulation. | ||
| - | |||
| - | <scene name='10/1078819/Ferritin_with_iron/1'>Ferritin with iron ion</scene> | ||
== Mechanism == | == Mechanism == | ||
Revision as of 23:32, 30 April 2025
Ferritin
| |||||||||||
References
- ↑ Carmona, F., Palacios, Ò., Gálvez, N., Cuesta, R., Atrian, S., Capdevila, M., & Domínguez-Vera, J. M. (n.d.). Ferritin iron uptake and release in the presence of metals and metalloproteins: Chemical implications in the brain.
- ↑ Knovich, M. A.; Storey, J. A.; Coffman, L. G.; Torti, S. V. Ferritin for the Clinician. Blood Rev 2009, 23 (3), 95–104.
- ↑ Bradley, J. M.; Le Brun, N. E.; Moore, G. R. Ferritins: Furnishing Proteins with Iron. JBIC Journal of Biological Inorganic Chemistry 2016, 21 (1), 13–28.
- ↑ 4.0 4.1 Srivastava, A.K., Reutovich, A.A., Hunter, N.J. et al. Ferritin microheterogeneity, subunit composition, functional, and physiological implications. Sci Rep 13, 19862 (2023). https://doi.org/10.1038/s41598-023-46880-9
- ↑ 5.0 5.1 Levi, S., & Rovida, E. (2015). Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiology of disease, 81, 134–143. https://doi.org/10.1016/j.nbd.2015.02.007
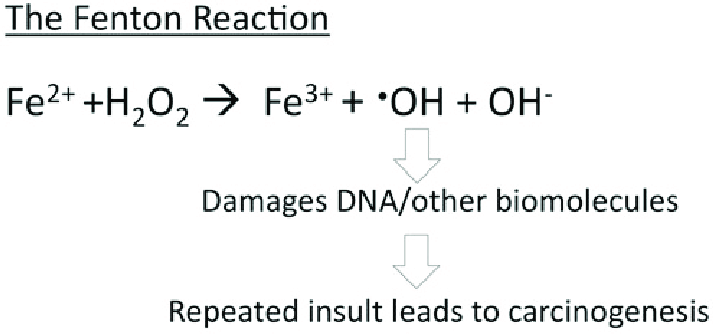
- ↑ 6.0 6.1 Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014
- ↑ 7.0 7.1 Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257
- ↑ Takahashi, T., & Kuyucak, S. (2003). Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophysical journal, 84(4), 2256–2263. https://doi.org/10.1016/S0006-3495(03)75031-0
- ↑ 9.0 9.1 https://chem.libretexts.org/Courses/Duke_University/Textbook%3A_Modern_Applications_of_Chemistry_(Cox)/10%3A_Bioinorganic_Chemistry/10.04%3A_Iron_Storage-_Ferritin
- ↑ Bou-Abdallah F. (2010). The iron redox and hydrolysis chemistry of the ferritins. Biochimica et biophysica acta, 1800(8), 719–731. https://doi.org/10.1016/j.bbagen.2010.03.021
- ↑ 11.0 11.1 11.2 11.3 https://www.thebloodproject.com/cases-archive/the-abcs-of-ferritin/how-does-iron-get-into-and-out-of-ferritin/#:~:text=Iron%20enters%20ferritin%20through%20pores,lysosomes%20%E2%80%93%20a%20process%20called%20ferritinophagy
- ↑ Wang, J., Wu, N., Peng, M. et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. 9, 463 (2023). https://doi.org/10.1038/s41420-023-01753-y
- ↑ Boss, M. A., & Chris Hammel, P. (2012). The role of diffusion in ferritin-induced relaxation enhancement of protons. Journal of magnetic resonance (San Diego, Calif. : 1997), 217, 36–40. https://doi.org/10.1016/j.jmr.2012.02.005
- ↑ Kotla, N. K., Dutta, P., Parimi, S., & Das, N. K. (2022). The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites, 12(7), 609. https://doi.org/10.3390/metabo12070609
- ↑ Liu, J. L., Fan, Y. G., Yang, Z. S., Wang, Z. Y., & Guo, C. (2018). Iron and Alzheimer's Disease: From Pathogenesis to Therapeutic Implications. Frontiers in neuroscience, 12, 632. https://doi.org/10.3389/fnins.2018.00632