We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Alexander Grayzel/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
== Mechanism == | == Mechanism == | ||
=== Iron Storage === | === Iron Storage === | ||
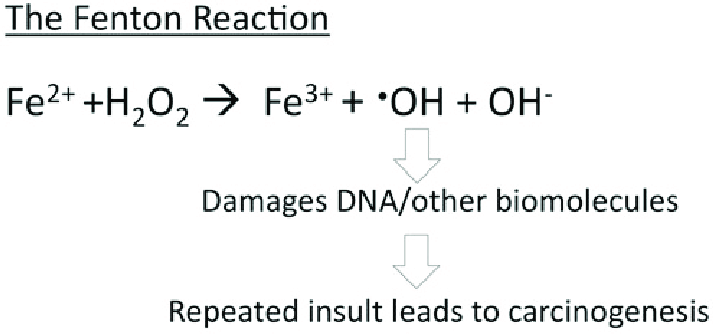
| - | Ferritin acts as an iron delivery vehicle which <scene name='10/1078819/Ferritin_with_iron/1'>brings in the Fe²⁺</scene> form of iron | + | Ferritin acts as an iron delivery vehicle which <scene name='10/1078819/Ferritin_with_iron/1'>brings in the Fe²⁺</scene> form of iron into its core. Iron then enters ferritin through ion channels. Going into further detail, Fe²⁺ ions are brought into the core through the <scene name='10/1078819/3-fold_channel_of_ferritin/1'>3-fold channel</scene> formed by the subunits of ferritin.<ref>Takahashi, T., & Kuyucak, S. (2003). Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophysical journal, 84(4), 2256–2263. https://doi.org/10.1016/S0006-3495(03)75031-0</ref> As stated previously, the 3-fold channels are comprised primarily of aspartate and glutamate residues which makes the pore hydrophilic. This hydrophilicity allows for diffusion of water, metal cations, and hydrophilic molecules into the core. The 3-fold channel is hypothesized to be the main channel for iron entering the core. On the other hand, the <scene name='10/1078819/4-fold_channel_of_ferritin/2'>4-fold channels</scene> are responsible for diffusion of oxygen and hydrogen peroxide, not iron.<ref name="LibreText">https://chem.libretexts.org/Courses/Duke_University/Textbook%3A_Modern_Applications_of_Chemistry_(Cox)/10%3A_Bioinorganic_Chemistry/10.04%3A_Iron_Storage-_Ferritin</ref> It is lined with non-polar residues such as leucine and ultimately makes the channel hydrophobic. This hydrophobicity allows for diffusion of oxygen and hydrogen peroxide into and out of the ferritin core. |
From a hard-soft acid-base (HSAB) perspective, this behavior is chemically intuitive. According to HSAB theory, hard acids prefer to bind with hard bases, and soft acids with soft bases.<ref name="Lopachin">Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257</ref> Fe³⁺ is a hard Lewis acid because it is small, highly charged, and not very polarizable. Ferritin’s iron-binding sites are rich in hard base residues such as glutamate and aspartate, which have oxygen donor atoms (hard bases).<ref name="Lopachin" /> This makes the iron-glutamate/aspartate interactions highly favorable, stabilizing Fe³⁺ in the protein’s core. In contrast, Fe²⁺ is a borderline acid and is more reactive, converting it to Fe³⁺ reduces the risk of it catalyzing the harmful Fenton reactions. | From a hard-soft acid-base (HSAB) perspective, this behavior is chemically intuitive. According to HSAB theory, hard acids prefer to bind with hard bases, and soft acids with soft bases.<ref name="Lopachin">Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257</ref> Fe³⁺ is a hard Lewis acid because it is small, highly charged, and not very polarizable. Ferritin’s iron-binding sites are rich in hard base residues such as glutamate and aspartate, which have oxygen donor atoms (hard bases).<ref name="Lopachin" /> This makes the iron-glutamate/aspartate interactions highly favorable, stabilizing Fe³⁺ in the protein’s core. In contrast, Fe²⁺ is a borderline acid and is more reactive, converting it to Fe³⁺ reduces the risk of it catalyzing the harmful Fenton reactions. | ||
Revision as of 11:58, 1 May 2025
Ferritin
| |||||||||||
References
- ↑ Carmona, F., Palacios, Ò., Gálvez, N., Cuesta, R., Atrian, S., Capdevila, M., & Domínguez-Vera, J. M. (n.d.). Ferritin iron uptake and release in the presence of metals and metalloproteins: Chemical implications in the brain.
- ↑ Knovich, M. A.; Storey, J. A.; Coffman, L. G.; Torti, S. V. Ferritin for the Clinician. Blood Rev 2009, 23 (3), 95–104.
- ↑ Bradley, J. M.; Le Brun, N. E.; Moore, G. R. Ferritins: Furnishing Proteins with Iron. JBIC Journal of Biological Inorganic Chemistry 2016, 21 (1), 13–28.
- ↑ 4.0 4.1 Srivastava, A.K., Reutovich, A.A., Hunter, N.J. et al. Ferritin microheterogeneity, subunit composition, functional, and physiological implications. Sci Rep 13, 19862 (2023). https://doi.org/10.1038/s41598-023-46880-9
- ↑ 5.0 5.1 Levi, S., & Rovida, E. (2015). Neuroferritinopathy: From ferritin structure modification to pathogenetic mechanism. Neurobiology of disease, 81, 134–143. https://doi.org/10.1016/j.nbd.2015.02.007
- ↑ Takahashi, T., & Kuyucak, S. (2003). Functional properties of threefold and fourfold channels in ferritin deduced from electrostatic calculations. Biophysical journal, 84(4), 2256–2263. https://doi.org/10.1016/S0006-3495(03)75031-0
- ↑ 7.0 7.1 https://chem.libretexts.org/Courses/Duke_University/Textbook%3A_Modern_Applications_of_Chemistry_(Cox)/10%3A_Bioinorganic_Chemistry/10.04%3A_Iron_Storage-_Ferritin
- ↑ 8.0 8.1 Lopachin, R. M., Gavin, T., Decaprio, A., & Barber, D. S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chemical research in toxicology, 25(2), 239–251. https://doi.org/10.1021/tx2003257
- ↑ Bou-Abdallah F. (2010). The iron redox and hydrolysis chemistry of the ferritins. Biochimica et biophysica acta, 1800(8), 719–731. https://doi.org/10.1016/j.bbagen.2010.03.021
- ↑ 10.0 10.1 Bystrom, L. M., Guzman, M. L., & Rivella, S. (2014). Iron and reactive oxygen species: friends or foes of cancer cells?. Antioxidants & redox signaling, 20(12), 1917–1924. https://doi.org/10.1089/ars.2012.5014
- ↑ 11.0 11.1 Tosha, T., Hasan, M. R., & Theil, E. C. (2008). The ferritin Fe2 site at the diiron catalytic center controls the reaction with O2 in the rapid mineralization pathway. Proceedings of the National Academy of Sciences of the United States of America, 105(47), 18182–18187. https://doi.org/10.1073/pnas.0805083105
- ↑ 12.0 12.1 12.2 12.3 https://www.thebloodproject.com/cases-archive/the-abcs-of-ferritin/how-does-iron-get-into-and-out-of-ferritin/#:~:text=Iron%20enters%20ferritin%20through%20pores,lysosomes%20%E2%80%93%20a%20process%20called%20ferritinophagy
- ↑ Wang, J., Wu, N., Peng, M. et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. 9, 463 (2023). https://doi.org/10.1038/s41420-023-01753-y
- ↑ Boss, M. A., & Chris Hammel, P. (2012). The role of diffusion in ferritin-induced relaxation enhancement of protons. Journal of magnetic resonance (San Diego, Calif. : 1997), 217, 36–40. https://doi.org/10.1016/j.jmr.2012.02.005
- ↑ Kotla, N. K., Dutta, P., Parimi, S., & Das, N. K. (2022). The Role of Ferritin in Health and Disease: Recent Advances and Understandings. Metabolites, 12(7), 609. https://doi.org/10.3390/metabo12070609
- ↑ Liu, J. L., Fan, Y. G., Yang, Z. S., Wang, Z. Y., & Guo, C. (2018). Iron and Alzheimer's Disease: From Pathogenesis to Therapeutic Implications. Frontiers in neuroscience, 12, 632. https://doi.org/10.3389/fnins.2018.00632