We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Vinícius M. Neto/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
Fibroins are fibrous structural proteins composed of multiple subunits that assemble into high-strength materials like silk and byssal threads. The exact composition varies by organism but typically features core fibroin filaments bundled within a sericin coating. These core filaments consist of three key components: a heavy chain (FibH), a light chain (FibL), and glycoproteins, all stabilized by disulfide bonds. The N-terminal domain of FibH (FibNT) plays a crucial role in the pH-dependent assembly of fibroin molecules during fiber formation <ref>DOI 10.1016/j.jmb.2012.02.040</ref>. | Fibroins are fibrous structural proteins composed of multiple subunits that assemble into high-strength materials like silk and byssal threads. The exact composition varies by organism but typically features core fibroin filaments bundled within a sericin coating. These core filaments consist of three key components: a heavy chain (FibH), a light chain (FibL), and glycoproteins, all stabilized by disulfide bonds. The N-terminal domain of FibH (FibNT) plays a crucial role in the pH-dependent assembly of fibroin molecules during fiber formation <ref>DOI 10.1016/j.jmb.2012.02.040</ref>. | ||
| + | == Biological production == | ||
| - | + | Bombyx mori fifth-instar larvae produce silk proteins in specialized silk glands, where synthesis is spatially organized - fibroins (FibH, FibL, and glycoproteins) are produced in the posterior silk gland while sericins are synthesized in the middle section. Both components are stored in the lumen of the middle silk gland prior to fiber formation. The spinning process is mediated by precise pH and ion concentration gradients along the anterior silk gland. As the silk proteins encounter this gradient, the FibNT domain undergoes a critical conformational transition from random coil to β-sheet structure. This pH-dependent structural change, driven by protonation of key acidic residues, confers stability and strength to the fiber while enabling the final assembly of mature silk<ref>DOI 10.1016/j.jmb.2012.02.040</ref>. | |
| - | + | ||
== Basic structure == | == Basic structure == | ||
| Line 22: | Line 22: | ||
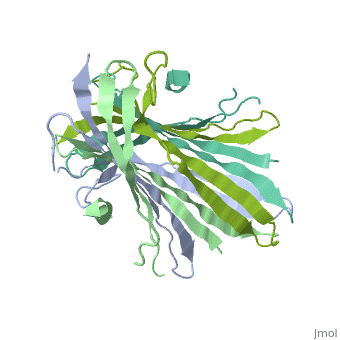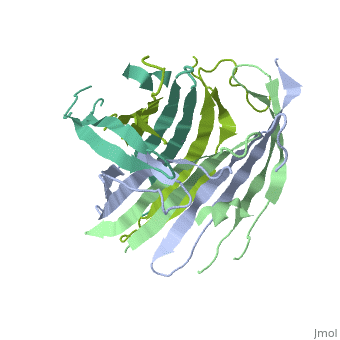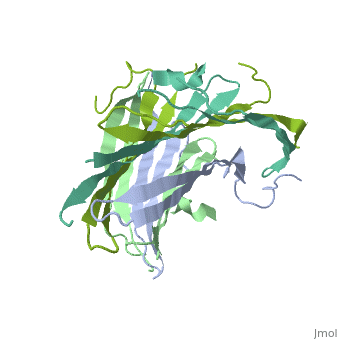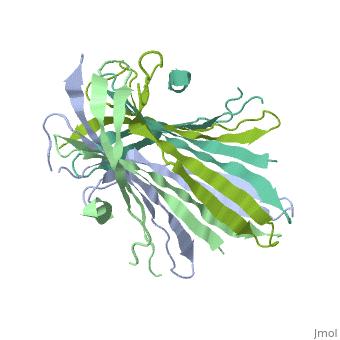
The FibNT homodimer exhibits the following <scene name='10/1082417/Beta_hairpins/1'>topology</scene>: β1<sub>A</sub>–β2<sub>A</sub>–β4<sub>B</sub>–β3<sub>B</sub>–β3<sub>A</sub>–β4<sub>A</sub>–β2<sub>B</sub>–β1<sub>B</sub>, where there are two <span style="background-color:black; color:yellow;">'''β-hairpins'''</span> (Thr36–Asn65 and Glu78–Ser107) connected with two <span style="background-color:black; color:cyan;">'''type I β-turns'''</span> (Asp49–Gly52 and Asp89–Gly92). The entire assembly is stabilized by an extensive network of <scene name='10/1082417/Hbondsv2/2'>hydrogen bonds</scene> between adjacent β-strands. | The FibNT homodimer exhibits the following <scene name='10/1082417/Beta_hairpins/1'>topology</scene>: β1<sub>A</sub>–β2<sub>A</sub>–β4<sub>B</sub>–β3<sub>B</sub>–β3<sub>A</sub>–β4<sub>A</sub>–β2<sub>B</sub>–β1<sub>B</sub>, where there are two <span style="background-color:black; color:yellow;">'''β-hairpins'''</span> (Thr36–Asn65 and Glu78–Ser107) connected with two <span style="background-color:black; color:cyan;">'''type I β-turns'''</span> (Asp49–Gly52 and Asp89–Gly92). The entire assembly is stabilized by an extensive network of <scene name='10/1082417/Hbondsv2/2'>hydrogen bonds</scene> between adjacent β-strands. | ||
| - | |||
| - | |||
== pH-Dependent Structural Transition == | == pH-Dependent Structural Transition == | ||
Revision as of 02:32, 19 June 2025
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ doi: https://dx.doi.org/10.1201/9781420015270
- ↑ doi: https://dx.doi.org/10.1038/nprot.2011.379
- ↑ He YX, Zhang NN, Li WF, Jia N, Chen BY, Zhou K, Zhang J, Chen Y, Zhou CZ. N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease. J Mol Biol. 2012 Mar 1. PMID:22387468 doi:10.1016/j.jmb.2012.02.040
- ↑ He YX, Zhang NN, Li WF, Jia N, Chen BY, Zhou K, Zhang J, Chen Y, Zhou CZ. N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease. J Mol Biol. 2012 Mar 1. PMID:22387468 doi:10.1016/j.jmb.2012.02.040