We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Vinícius M. Neto/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
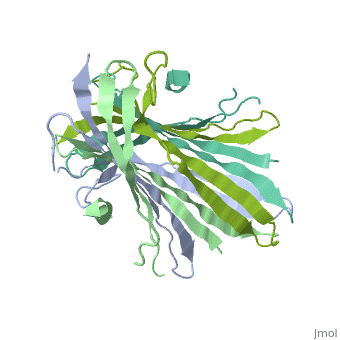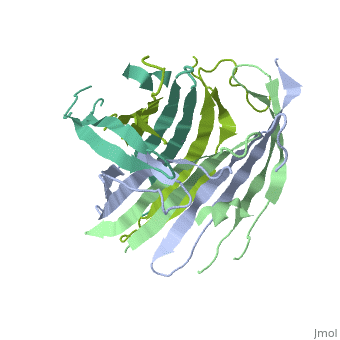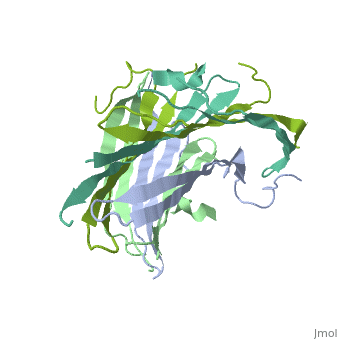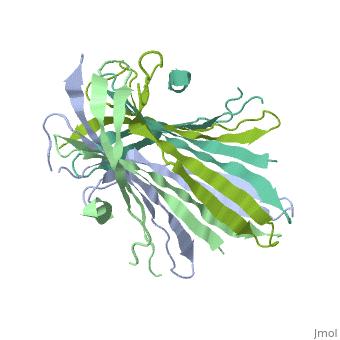
| - | The FibNT homodimer exhibits the following <scene name='10/1082417/Beta_hairpins/2'>topology</scene>: β1<sub>A</sub>–β2<sub>A</sub>–β4<sub>B</sub>–β3<sub>B</sub>–β3<sub>A</sub>–β4<sub>A</sub>–β2<sub>B</sub>–β1<sub>B</sub>, where there are two <span style="background-color:black; color:yellow;">'''β-hairpins'''</span> (Thr36–Asn65 and Glu78–Ser107) connected with two <span style="background-color:black; color:cyan;">'''type I β-turns'''</span> (Asp49–Gly52 and Asp89–Gly92) for each monomer (animation highlights the '''chain A'''). The entire assembly is stabilized by an extensive network of <scene name='10/1082417/Hbondsv2/2'>hydrogen bonds</scene> between adjacent β-strands. | + | The FibNT homodimer exhibits the following <scene name='10/1082417/Beta_hairpins/2'>topology</scene>: β1<sub>A</sub>–β2<sub>A</sub>–β4<sub>B</sub>–β3<sub>B</sub>–β3<sub>A</sub>–β4<sub>A</sub>–β2<sub>B</sub>–β1<sub>B</sub>, where there are two <span style="background-color:black; color:yellow;">'''β-hairpins'''</span> (Thr36–Asn65 and Glu78–Ser107) connected with two <span style="background-color:black; color:cyan;">'''type I β-turns'''</span> (Asp49–Gly52 and Asp89–Gly92) for each monomer (animation highlights the '''chain A'''). The entire assembly is stabilized by an extensive network of <scene name='10/1082417/Hbondsv2/2'>hydrogen bonds</scene> between adjacent β-strands<ref name="PDB">DOI 10.1016/j.jmb.2012.02.040</ref>. |
== pH-Dependent Structural Transition == | == pH-Dependent Structural Transition == | ||
| Line 26: | Line 26: | ||
The structure of fibroin is highly pH-dependent. During the natural silk-spinning process, the fibroin solution experiences a steep pH gradient along the silk gland (from anterior to posterior), which triggers the gelation of condensed fibroin. Specifically, the N-terminal domain (FibNT) remains in a disordered random-coil state at neutral pH, preventing premature β-sheet formation. Only when the pH drops to approximately 6.0 does FibNT undergo a cooperative structural transition, adopting the stable β-sheet conformation essential for fiber assembly. | The structure of fibroin is highly pH-dependent. During the natural silk-spinning process, the fibroin solution experiences a steep pH gradient along the silk gland (from anterior to posterior), which triggers the gelation of condensed fibroin. Specifically, the N-terminal domain (FibNT) remains in a disordered random-coil state at neutral pH, preventing premature β-sheet formation. Only when the pH drops to approximately 6.0 does FibNT undergo a cooperative structural transition, adopting the stable β-sheet conformation essential for fiber assembly. | ||
| - | Interactions between acidic residues in FibNT are critical for pH-sensitive behavior. Near the transition point (pH ~6.0), some residues exhibit up-shifted pK<sub>a</sub> values, allowing them to remain ionized at neutral pH. This sustained negative charge creates electrostatic repulsion, actively preventing premature folding and β-sheet assembly. For example, at higher pH, <scene name='10/1082417/Essential_h_bonds/2'>key hydrogen bonds</scene>—such as those between <span style="background-color:black; color:yellow;">'''Glu56–Asp44'''</span> and <span style="background-color:black; color:cyan;">'''Asp100–Glu98'''</span>—are disrupted, destabilizing β-sheet conformations until protonation occurs at lower pH. | + | Interactions between acidic residues in FibNT are critical for pH-sensitive behavior. Near the transition point (pH ~6.0), some residues exhibit up-shifted pK<sub>a</sub> values, allowing them to remain ionized at neutral pH. This sustained negative charge creates electrostatic repulsion, actively preventing premature folding and β-sheet assembly. For example, at higher pH, <scene name='10/1082417/Essential_h_bonds/2'>key hydrogen bonds</scene>—such as those between <span style="background-color:black; color:yellow;">'''Glu56–Asp44'''</span> and <span style="background-color:black; color:cyan;">'''Asp100–Glu98'''</span>—are disrupted, destabilizing β-sheet conformations until protonation occurs at lower pH<ref name="PDB">DOI 10.1016/j.jmb.2012.02.040</ref>. |
</StructureSection> | </StructureSection> | ||
Revision as of 17:07, 19 June 2025
FibNT (3UA0)
| |||||||||||
References
- ↑ doi: https://dx.doi.org/10.1201/9781420015270
- ↑ doi: https://dx.doi.org/10.1038/nprot.2011.379
- ↑ 3.0 3.1 3.2 3.3 He YX, Zhang NN, Li WF, Jia N, Chen BY, Zhou K, Zhang J, Chen Y, Zhou CZ. N-Terminal Domain of Bombyx mori Fibroin Mediates the Assembly of Silk in Response to pH Decrease. J Mol Biol. 2012 Mar 1. PMID:22387468 doi:10.1016/j.jmb.2012.02.040