We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Thioredoxin
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='' size='350' side='right' caption='Human thioredoxin (PDB entry [[1ert]])' scene='43/430885/Cv/2'> | <StructureSection load='' size='350' side='right' caption='Human thioredoxin (PDB entry [[1ert]])' scene='43/430885/Cv/2'> | ||
| - | == Function == | + | == Function =={{Heading|Thioredoxin Reductase in Fungal Pathogens: A Drug Target Perspective}} |
| + | |||
| + | In response to the toxicity of reactive oxygen species (ROS), pathogenic organisms such as fungi possess enzymatic defense mechanisms. One such enzyme is Thioredoxin Reductase (TrxR), which plays essential roles in several biological processes including DNA synthesis, redox signaling, and oxidative stress response. Inhibiting TrxR can weaken pathogens, which may have significant implications for public health and agriculture. | ||
| + | |||
| + | Notably, fungal TrxRs are structurally and biochemically distinct from those found in mammals — a desirable feature for drug development efforts (Oliveira, 2010). Furthermore, fungal TrxRs do not reduce the host thioredoxin, supporting their relevance as selective drug targets. | ||
| + | |||
| + | === The Thioredoxin System === | ||
| + | The thioredoxin system is composed of two main proteins: Thioredoxin (Trx) and Thioredoxin Reductase (TrxR), along with NADPH as a cofactor. This system functions primarily by reducing antioxidant proteins such as Peroxiredoxins (Prx) and Methionine Sulfoxide Reductases. It is also involved in other redox regulatory functions. | ||
| + | |||
| + | Historically, Trxs were the first enzymes identified as responsible for reducing ribonucleotides to deoxyribonucleotides in *Escherichia coli*, an essential step in DNA synthesis (Laurent et al., 1964). | ||
| + | |||
| + | === Mechanism of Action === | ||
| + | TrxR catalyzes the reduction of the disulfide bond in the active site of Trx, enabling Trx to catalytically reduce target proteins. TrxR is a flavoprotein, using NADPH as the terminal electron donor. | ||
| + | |||
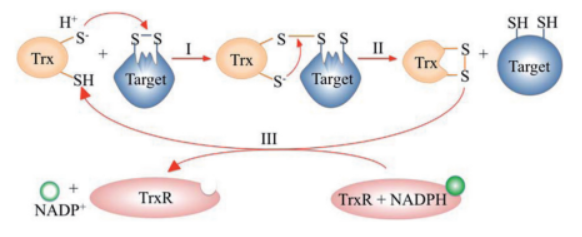
| + | The mechanism proceeds in sequential thiol-disulfide exchange reactions: | ||
| + | |||
| + | * **Step I:** The N-terminal cysteine of Trx attacks a disulfide bond in the target protein, forming a mixed disulfide intermediate. | ||
| + | * **Step II:** The C-terminal cysteine of Trx resolves the intermediate, releasing the reduced protein and generating oxidized Trx. | ||
| + | * **Step III:** TrxR recycles Trx using electrons from NADPH (Netto et al., 2015). | ||
| + | |||
| + | [[Image:Thioredoxin_cycle.png|600px]] | ||
| + | ''Figure 1: Catalytic cycle of the thioredoxin system. Adapted from Netto et al., 2015.'' | ||
| + | |||
| + | |||
| + | === References === | ||
| + | * Holmgren, A. (2000). Antioxidant function of thioredoxin and glutaredoxin systems. *Antioxidants & Redox Signaling, 2*(4), 811–820. | ||
| + | * Oliveira, L.O. (2010). [Relevant source on fungal TrxR properties]. | ||
| + | * Laurent, T. C., Moore, E. C., & Reichard, P. (1964). Enzymatic synthesis of deoxyribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from *Escherichia coli*. *Journal of Biological Chemistry*, 239(10), 3436–3444. | ||
| + | * Netto, L.E.S. et al. (2015). [Thioredoxin system cycle and structural biology]. | ||
| + | |||
| + | {{Stub|This article is a draft. Please contribute to improve its accuracy and completeness.}} | ||
| + | |||
[[Thioredoxin]] (Trx) is an enzyme which facilitates, in its reduced form, the reduction of proteins by cysteine thiol-disulfide exchange<ref>PMID:3152490</ref>. They contain a CXXC motif.<br /> | [[Thioredoxin]] (Trx) is an enzyme which facilitates, in its reduced form, the reduction of proteins by cysteine thiol-disulfide exchange<ref>PMID:3152490</ref>. They contain a CXXC motif.<br /> | ||
* '''Trx-1''' is a mammalian cellular protein.<br /> | * '''Trx-1''' is a mammalian cellular protein.<br /> | ||
Revision as of 12:27, 23 June 2025
| |||||||||||
References
- ↑ Gleason FK, Holmgren A. Thioredoxin and related proteins in procaryotes. FEMS Microbiol Rev. 1988 Dec;4(4):271-97. PMID:3152490
- ↑ Sumida Y, Nakashima T, Yoh T, Furutani M, Hirohama A, Kakisaka Y, Nakajima Y, Ishikawa H, Mitsuyoshi H, Okanoue T, Kashima K, Nakamura H, Yodoi J. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003 Jan;38(1):32-8. PMID:12480557
- ↑ Burke-Gaffney A, Callister ME, Nakamura H. Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci. 2005 Aug;26(8):398-404. PMID:15990177 doi:http://dx.doi.org/10.1016/j.tips.2005.06.005
- ↑ Weichsel A, Gasdaska JR, Powis G, Montfort WR. Crystal structures of reduced, oxidized, and mutated human thioredoxins: evidence for a regulatory homodimer. Structure. 1996 Jun 15;4(6):735-51. PMID:8805557
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Laura Maria Batista Leal, Joel L. Sussman