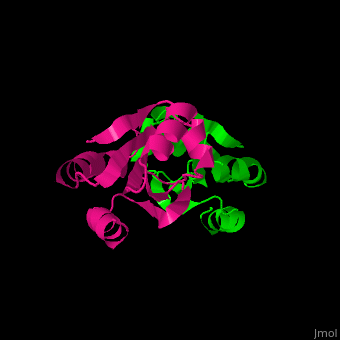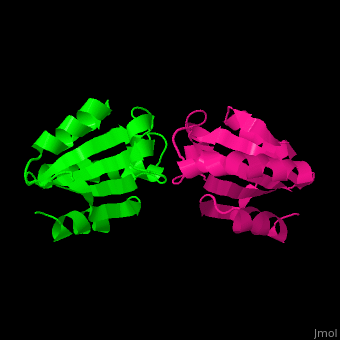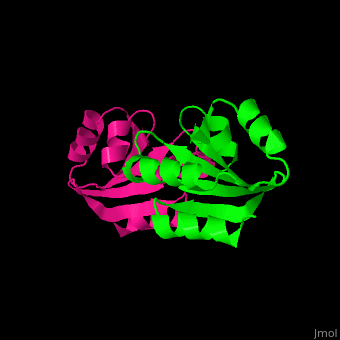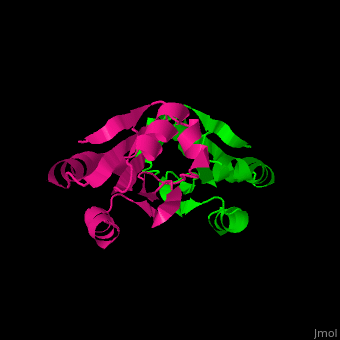We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Thioredoxin
From Proteopedia
(Difference between revisions)
| Line 40: | Line 40: | ||
== Structural highlights == | == Structural highlights == | ||
| + | <StructureSection load="1ert" size="400" side="right" caption="Human thioredoxin (PDB entry 1ERT)"> | ||
| + | <script> | ||
| + | stage.loadFile("rcsb://1ert", {defaultRepresentation: true}).then(function(o){ | ||
| + | |||
| + | // Remove a representação padrão | ||
| + | o.removeAllRepresentations(); | ||
| + | |||
| + | // Colorir hélices α de vermelho | ||
| + | o.addRepresentation("cartoon", { | ||
| + | sele: "helix", | ||
| + | color: "red" | ||
| + | }); | ||
| + | |||
| + | // Colorir folhas β de azul | ||
| + | o.addRepresentation("cartoon", { | ||
| + | sele: "sheet", | ||
| + | color: "blue" | ||
| + | }); | ||
| + | |||
| + | // Exibir o restante da proteína em cinza claro | ||
| + | o.addRepresentation("cartoon", { | ||
| + | sele: "protein and not (helix or sheet)", | ||
| + | color: "lightgrey" | ||
| + | }); | ||
| + | |||
| + | stage.autoView(); | ||
| + | }); | ||
| + | </script> | ||
| + | </StructureSection> | ||
| + | |||
| + | <p> | ||
| + | All thioredoxin proteins share a common structure, consisting of <b>four α-helices</b> (highlighted in red) and <b>five β-sheets</b> (highlighted in blue). This conserved fold is crucial for the redox activity of thioredoxins. | ||
| + | </p> | ||
| + | |||
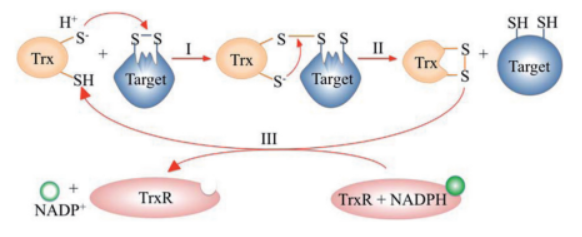
The <scene name='43/430885/Cv/4'>active site motif Cys-Gly-Pro-Cys</scene> is involved in the reduction of disulfide bonds in proteins<ref>Åslund F et al. (1997). J Biol Chem. 272(48):30780–30786.</ref>. | The <scene name='43/430885/Cv/4'>active site motif Cys-Gly-Pro-Cys</scene> is involved in the reduction of disulfide bonds in proteins<ref>Åslund F et al. (1997). J Biol Chem. 272(48):30780–30786.</ref>. | ||
Unlike many other thioredoxins, the human cytoplasmic thioredoxin has three cysteine residues (Cys 62, Cys 69, Cys 73) additional to the active site <scene name='43/430885/Cv/2'>Cys 32 and Cys 35</scene>. | Unlike many other thioredoxins, the human cytoplasmic thioredoxin has three cysteine residues (Cys 62, Cys 69, Cys 73) additional to the active site <scene name='43/430885/Cv/2'>Cys 32 and Cys 35</scene>. | ||
Revision as of 19:41, 30 June 2025
| |||||||||||
All thioredoxin proteins share a common structure, consisting of four α-helices (highlighted in red) and five β-sheets (highlighted in blue). This conserved fold is crucial for the redox activity of thioredoxins.
The is involved in the reduction of disulfide bonds in proteins[8]. Unlike many other thioredoxins, the human cytoplasmic thioredoxin has three cysteine residues (Cys 62, Cys 69, Cys 73) additional to the active site . The human cytoplasmic thioredoxin crystal structure reveals a homodimer with .
3D Structures of Thioredoxin
</StructureSection>
References
- ↑ Oliveira LO. (2010). Caracterização de tiorredoxinas de fungos filamentosos como alvos moleculares para drogas antifúngicas. Tese de doutorado, Universidade de São Paulo.
- ↑ Laurent TC, Moore EC, Reichard P. (1964). J Biol Chem. 239(10):3436–3444.
- ↑ Netto LES et al. (2015). Free Radic Biol Med. 89:60–75.
- ↑ Holmgren A. (2000). Antioxid Redox Signal. 2(4):811–820.
- ↑ Sies H. (1985). Oxidative stress: introductory remarks. Academic Press.
- ↑ Yamada G et al. (2003). J Hepatol. 38(1):32–38.
- ↑ Arnér ESJ, Holmgren A. (2006). Semin Cancer Biol. 16(6):420–426.
- ↑ Åslund F et al. (1997). J Biol Chem. 272(48):30780–30786.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Laura Maria Batista Leal, Joel L. Sussman