We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
1wki
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | [[Image:1wki. | + | {{Seed}} |
| + | [[Image:1wki.png|left|200px]] | ||
<!-- | <!-- | ||
| Line 9: | Line 10: | ||
{{STRUCTURE_1wki| PDB=1wki | SCENE= }} | {{STRUCTURE_1wki| PDB=1wki | SCENE= }} | ||
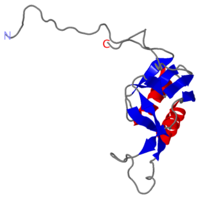
| - | + | ===solution structure of ribosomal protein L16 from thermus thermophilus HB8=== | |
| - | + | <!-- | |
| - | + | The line below this paragraph, {{ABSTRACT_PUBMED_15561149}}, adds the Publication Abstract to the page | |
| + | (as it appears on PubMed at http://www.pubmed.gov), where 15561149 is the PubMed ID number. | ||
| + | --> | ||
| + | {{ABSTRACT_PUBMED_15561149}} | ||
==About this Structure== | ==About this Structure== | ||
| - | Full | + | Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1WKI OCA]. |
==Reference== | ==Reference== | ||
| Line 34: | Line 38: | ||
[[Category: Rsgi]] | [[Category: Rsgi]] | ||
[[Category: Structural genomic]] | [[Category: Structural genomic]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | |
| + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Mon Jul 28 20:32:42 2008'' | ||
Revision as of 17:32, 28 July 2008
solution structure of ribosomal protein L16 from thermus thermophilus HB8
Template:ABSTRACT PUBMED 15561149
About this Structure
Full experimental information is available from OCA.
Reference
Solution structure of ribosomal protein L16 from Thermus thermophilus HB8., Nishimura M, Yoshida T, Shirouzu M, Terada T, Kuramitsu S, Yokoyama S, Ohkubo T, Kobayashi Y, J Mol Biol. 2004 Dec 10;344(5):1369-83. PMID:15561149
Page seeded by OCA on Mon Jul 28 20:32:42 2008