Sandbox Aryan 20221057 BI3323-Aug2025
From Proteopedia
| Line 7: | Line 7: | ||
'''Key Interactions | '''Key Interactions | ||
''' | ''' | ||
| + | |||
Cas9's PI domain (residues ~1100-1368) mediates multiple contacts: electrostatic histone tail interactions (non-essential for binding), PI edge lysine K1155 stabilizing the post-cleavage complex, and core DNA loops (H1264, R1298, K1300) causing inhibitory non-specific binding. Mutants disrupting PI-core DNA contacts (H1264A/R1298Q) enhance both in vitro cleavage efficiency and rice genome editing. | Cas9's PI domain (residues ~1100-1368) mediates multiple contacts: electrostatic histone tail interactions (non-essential for binding), PI edge lysine K1155 stabilizing the post-cleavage complex, and core DNA loops (H1264, R1298, K1300) causing inhibitory non-specific binding. Mutants disrupting PI-core DNA contacts (H1264A/R1298Q) enhance both in vitro cleavage efficiency and rice genome editing. | ||
| | ||
| Line 13: | Line 14: | ||
'''Biological Insights''' | '''Biological Insights''' | ||
| - | Nucleosomes inhibit Cas9 via two mechanisms: (1) DNA end inflexibility blocks access; (2) PI domain trapping restricts domain motions needed for cleavage. Entry-exit asymmetry arises from Widom601 sequence-dependent flexibility, explaining variable editing efficiency across chromatin contexts. These findings reveal Cas9's eukaryotic adaptation and guide chromatin-optimized variants for improved genome editing.[[Image: | + | Nucleosomes inhibit Cas9 via two mechanisms: (1) DNA end inflexibility blocks access; (2) PI domain trapping restricts domain motions needed for cleavage. Entry-exit asymmetry arises from Widom601 sequence-dependent flexibility, explaining variable editing efficiency across chromatin contexts. These findings reveal Cas9's eukaryotic adaptation and guide chromatin-optimized variants for improved genome editing. |
| + | |||
| + | |||
| + | ''Scene 1: Overall complex'' | ||
| + | [[Image:8YNYOverall.jpg]] | ||
Revision as of 17:43, 30 November 2025
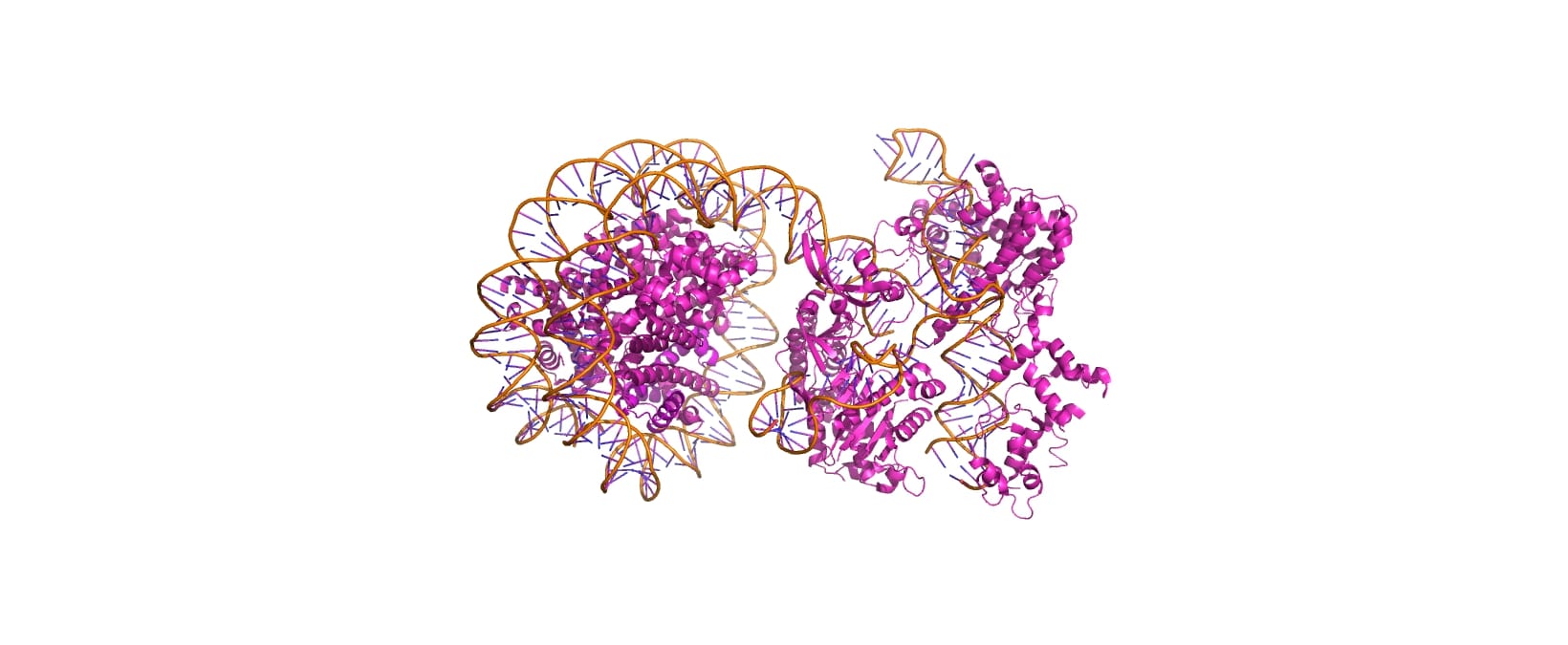
Cas9-Nucleosome Complex (PDB: 8YNY)
Cas9-sgRNA ribonucleoprotein targets linker DNA (PAM1/PAM28) and entry-exit regions (SHL6) of nucleosomes, avoiding tightly wrapped core DNA (SHL0-5), as revealed by native-PAGE on Widom 601 nucleosomes. The cryo-EM structure (PDB: 8YNY, 4.52 Å, EMDB: EMD-39431) captures the post-cleavage ternary complex at PAM1, showing ~15 bp DNA peeled from the histone octamer, exposing H3 N-terminus—mimicking eukaryotic nucleosome unwrapping.
Key Interactions
Cas9's PI domain (residues ~1100-1368) mediates multiple contacts: electrostatic histone tail interactions (non-essential for binding), PI edge lysine K1155 stabilizing the post-cleavage complex, and core DNA loops (H1264, R1298, K1300) causing inhibitory non-specific binding. Mutants disrupting PI-core DNA contacts (H1264A/R1298Q) enhance both in vitro cleavage efficiency and rice genome editing.
Biological Insights
Nucleosomes inhibit Cas9 via two mechanisms: (1) DNA end inflexibility blocks access; (2) PI domain trapping restricts domain motions needed for cleavage. Entry-exit asymmetry arises from Widom601 sequence-dependent flexibility, explaining variable editing efficiency across chromatin contexts. These findings reveal Cas9's eukaryotic adaptation and guide chromatin-optimized variants for improved genome editing.