1a0i
From Proteopedia
(New page: 200px<br /> <applet load="1a0i" size="450" color="white" frame="true" align="right" spinBox="true" caption="1a0i, resolution 2.6Å" /> '''ATP-DEPENDENT DNA LI...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1a0i.gif|left|200px]]<br /> | + | [[Image:1a0i.gif|left|200px]]<br /><applet load="1a0i" size="350" color="white" frame="true" align="right" spinBox="true" |
| - | <applet load="1a0i" size=" | + | |
caption="1a0i, resolution 2.6Å" /> | caption="1a0i, resolution 2.6Å" /> | ||
'''ATP-DEPENDENT DNA LIGASE FROM BACTERIOPHAGE T7 COMPLEX WITH ATP'''<br /> | '''ATP-DEPENDENT DNA LIGASE FROM BACTERIOPHAGE T7 COMPLEX WITH ATP'''<br /> | ||
==Overview== | ==Overview== | ||
| - | The crystal structure of the ATP-dependent DNA ligase from bacteriophage | + | The crystal structure of the ATP-dependent DNA ligase from bacteriophage T7 has been solved at 2.6 A resolution. The protein comprises two domains with a deep cleft running between them. The structure of a complex with ATP reveals that the nucleotide binding pocket is situated on the larger N-terminal domain, at the base of the cleft between the two domains of the enzyme. Comparison of the overall domain structure with that of DNA methyltransferases, coupled with other evidence, suggests that DNA also binds in this cleft. Since this structure is the first of the nucleotidyltransferase superfamily, which includes the eukaryotic mRNA capping enzymes, the relationship between the structure of DNA ligase and that of other nucleotidyltransferases is also discussed. |
==About this Structure== | ==About this Structure== | ||
| - | 1A0I is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Bacteriophage_t7 Bacteriophage t7] with ATP as [http://en.wikipedia.org/wiki/ligand ligand]. The following page contains interesting information on the relation of 1A0I with [[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb55_1.html DNA Ligase]]. Active as [http://en.wikipedia.org/wiki/DNA_ligase_(ATP) DNA ligase (ATP)], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=6.5.1.1 6.5.1.1] Full crystallographic information is available from [http:// | + | 1A0I is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Bacteriophage_t7 Bacteriophage t7] with <scene name='pdbligand=ATP:'>ATP</scene> as [http://en.wikipedia.org/wiki/ligand ligand]. The following page contains interesting information on the relation of 1A0I with [[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb55_1.html DNA Ligase]]. Active as [http://en.wikipedia.org/wiki/DNA_ligase_(ATP) DNA ligase (ATP)], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=6.5.1.1 6.5.1.1] Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1A0I OCA]. |
==Reference== | ==Reference== | ||
| Line 16: | Line 15: | ||
[[Category: DNA ligase (ATP)]] | [[Category: DNA ligase (ATP)]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| - | [[Category: Ashford, S | + | [[Category: Ashford, S R.]] |
| - | [[Category: Doherty, A | + | [[Category: Doherty, A J.]] |
| - | [[Category: Subramanya, H | + | [[Category: Subramanya, H S.]] |
| - | [[Category: Wigley, D | + | [[Category: Wigley, D B.]] |
[[Category: ATP]] | [[Category: ATP]] | ||
[[Category: dna replication]] | [[Category: dna replication]] | ||
[[Category: ligase]] | [[Category: ligase]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 11:39:34 2008'' |
Revision as of 09:39, 21 February 2008
|
ATP-DEPENDENT DNA LIGASE FROM BACTERIOPHAGE T7 COMPLEX WITH ATP
Overview
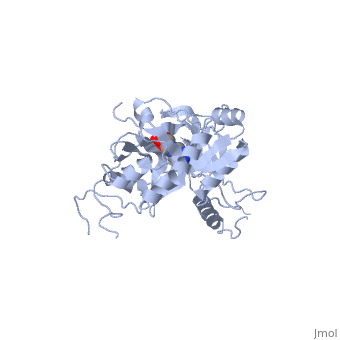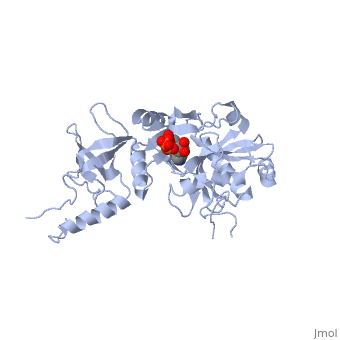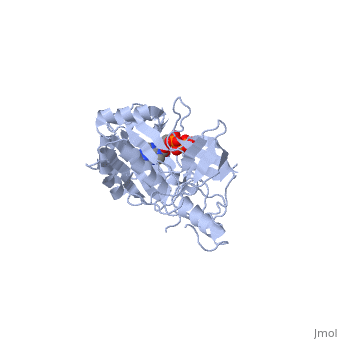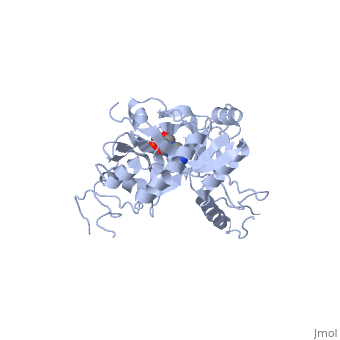
The crystal structure of the ATP-dependent DNA ligase from bacteriophage T7 has been solved at 2.6 A resolution. The protein comprises two domains with a deep cleft running between them. The structure of a complex with ATP reveals that the nucleotide binding pocket is situated on the larger N-terminal domain, at the base of the cleft between the two domains of the enzyme. Comparison of the overall domain structure with that of DNA methyltransferases, coupled with other evidence, suggests that DNA also binds in this cleft. Since this structure is the first of the nucleotidyltransferase superfamily, which includes the eukaryotic mRNA capping enzymes, the relationship between the structure of DNA ligase and that of other nucleotidyltransferases is also discussed.
About this Structure
1A0I is a Single protein structure of sequence from Bacteriophage t7 with as ligand. The following page contains interesting information on the relation of 1A0I with [DNA Ligase]. Active as DNA ligase (ATP), with EC number 6.5.1.1 Full crystallographic information is available from OCA.
Reference
Crystal structure of an ATP-dependent DNA ligase from bacteriophage T7., Subramanya HS, Doherty AJ, Ashford SR, Wigley DB, Cell. 1996 May 17;85(4):607-15. PMID:8653795
Page seeded by OCA on Thu Feb 21 11:39:34 2008