We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
4ald
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | [[Image:4ald. | + | [[Image:4ald.png|left|200px]] |
<!-- | <!-- | ||
| Line 12: | Line 12: | ||
| - | + | {{ABSTRACT_10048322}} | |
| - | + | ||
==About this Structure== | ==About this Structure== | ||
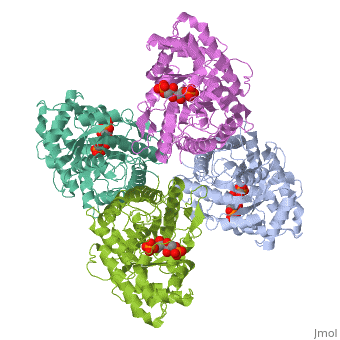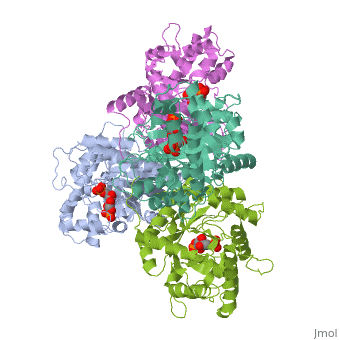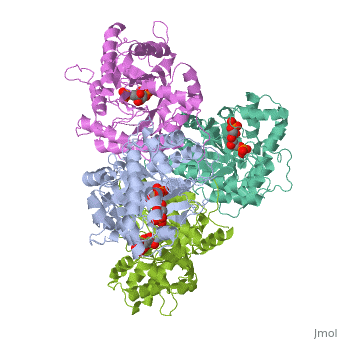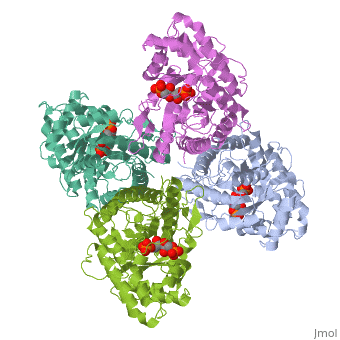
4ALD is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. The following page contains interesting information on the relation of 4ALD with [[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb50_1.html The Glycolytic Enzymes]]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4ALD OCA]. | 4ALD is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. The following page contains interesting information on the relation of 4ALD with [[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb50_1.html The Glycolytic Enzymes]]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4ALD OCA]. | ||
| - | |||
| - | ==Reference== | ||
| - | Crystal structure of human muscle aldolase complexed with fructose 1,6-bisphosphate: mechanistic implications., Dalby A, Dauter Z, Littlechild JA, Protein Sci. 1999 Feb;8(2):291-7. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10048322 10048322] | ||
[[Category: Fructose-bisphosphate aldolase]] | [[Category: Fructose-bisphosphate aldolase]] | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
| Line 30: | Line 26: | ||
[[Category: Tim barrel]] | [[Category: Tim barrel]] | ||
[[Category: Type 1 aldolase]] | [[Category: Type 1 aldolase]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | |
| + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Jun 26 18:28:26 2008'' | ||
Revision as of 15:28, 26 June 2008
| |||||||||
| 4ald, resolution 2.80Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Activity: | Fructose-bisphosphate aldolase, with EC number 4.1.2.13 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
HUMAN MUSCLE FRUCTOSE 1,6-BISPHOSPHATE ALDOLASE COMPLEXED WITH FRUCTOSE 1,6-BISPHOSPHATE
About this Structure
4ALD is a Single protein structure of sequence from Homo sapiens. The following page contains interesting information on the relation of 4ALD with [The Glycolytic Enzymes]. Full crystallographic information is available from OCA.
Page seeded by OCA on Thu Jun 26 18:28:26 2008