1qr0
From Proteopedia
(New page: 200px<br /><applet load="1qr0" size="450" color="white" frame="true" align="right" spinBox="true" caption="1qr0, resolution 1.90Å" /> '''CRYSTAL STRUCTURE OF...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1qr0.jpg|left|200px]]<br /><applet load="1qr0" size=" | + | [[Image:1qr0.jpg|left|200px]]<br /><applet load="1qr0" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="1qr0, resolution 1.90Å" /> | caption="1qr0, resolution 1.90Å" /> | ||
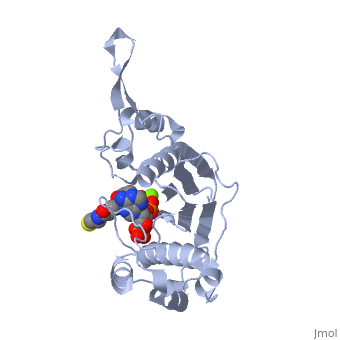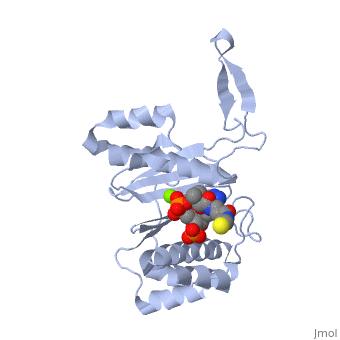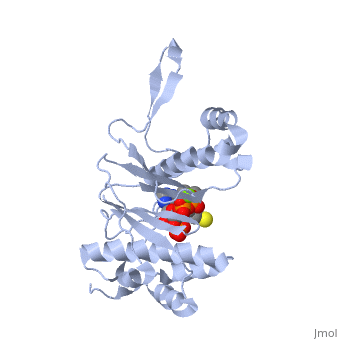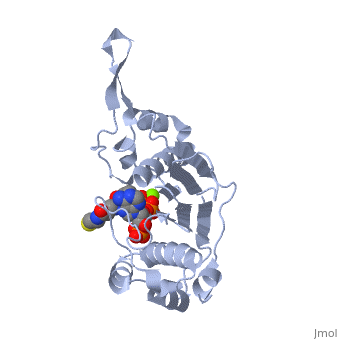
'''CRYSTAL STRUCTURE OF THE 4'-PHOSPHOPANTETHEINYL TRANSFERASE SFP-COENZYME A COMPLEX'''<br /> | '''CRYSTAL STRUCTURE OF THE 4'-PHOSPHOPANTETHEINYL TRANSFERASE SFP-COENZYME A COMPLEX'''<br /> | ||
==Overview== | ==Overview== | ||
| - | The Bacillus subtilis Sfp protein activates the peptidyl carrier protein | + | The Bacillus subtilis Sfp protein activates the peptidyl carrier protein (PCP) domains of surfactin synthetase by transferring the 4'-phosphopantetheinyl moiety of coenzyme A (CoA) to a serine residue conserved in all PCPs. Its wide PCP substrate spectrum renders Sfp a biotechnologically valuable enzyme for use in combinatorial non-ribosomal peptide synthesis. The structure of the Sfp-CoA complex determined at 1.8 A resolution reveals a novel alpha/beta-fold exhibiting an unexpected intramolecular 2-fold pseudosymmetry. This suggests a similar fold and dimerization mode for the homodimeric phosphopantetheinyl transferases such as acyl carrier protein synthase. The active site of Sfp accommodates a magnesium ion, which is complexed by the CoA pyrophosphate, the side chains of three acidic amino acids and one water molecule. CoA is bound in a fashion that differs in many aspects from all known CoA-protein complex structures. The structure reveals regions likely to be involved in the interaction with the PCP substrate. |
==About this Structure== | ==About this Structure== | ||
| - | 1QR0 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Bacillus_subtilis Bacillus subtilis] with MG and COA as [http://en.wikipedia.org/wiki/ligands ligands]. Full crystallographic information is available from [http:// | + | 1QR0 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Bacillus_subtilis Bacillus subtilis] with <scene name='pdbligand=MG:'>MG</scene> and <scene name='pdbligand=COA:'>COA</scene> as [http://en.wikipedia.org/wiki/ligands ligands]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1QR0 OCA]. |
==Reference== | ==Reference== | ||
| Line 14: | Line 14: | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Ficner, R.]] | [[Category: Ficner, R.]] | ||
| - | [[Category: Marahiel, A | + | [[Category: Marahiel, A M.]] |
| - | [[Category: Mofid, R | + | [[Category: Mofid, R M.]] |
[[Category: Reuter, K.]] | [[Category: Reuter, K.]] | ||
[[Category: COA]] | [[Category: COA]] | ||
| Line 21: | Line 21: | ||
[[Category: protein-coenzyme a complex]] | [[Category: protein-coenzyme a complex]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 14:42:42 2008'' |
Revision as of 12:42, 21 February 2008
|
CRYSTAL STRUCTURE OF THE 4'-PHOSPHOPANTETHEINYL TRANSFERASE SFP-COENZYME A COMPLEX
Overview
The Bacillus subtilis Sfp protein activates the peptidyl carrier protein (PCP) domains of surfactin synthetase by transferring the 4'-phosphopantetheinyl moiety of coenzyme A (CoA) to a serine residue conserved in all PCPs. Its wide PCP substrate spectrum renders Sfp a biotechnologically valuable enzyme for use in combinatorial non-ribosomal peptide synthesis. The structure of the Sfp-CoA complex determined at 1.8 A resolution reveals a novel alpha/beta-fold exhibiting an unexpected intramolecular 2-fold pseudosymmetry. This suggests a similar fold and dimerization mode for the homodimeric phosphopantetheinyl transferases such as acyl carrier protein synthase. The active site of Sfp accommodates a magnesium ion, which is complexed by the CoA pyrophosphate, the side chains of three acidic amino acids and one water molecule. CoA is bound in a fashion that differs in many aspects from all known CoA-protein complex structures. The structure reveals regions likely to be involved in the interaction with the PCP substrate.
About this Structure
1QR0 is a Single protein structure of sequence from Bacillus subtilis with and as ligands. Full crystallographic information is available from OCA.
Reference
Crystal structure of the surfactin synthetase-activating enzyme sfp: a prototype of the 4'-phosphopantetheinyl transferase superfamily., Reuter K, Mofid MR, Marahiel MA, Ficner R, EMBO J. 1999 Dec 1;18(23):6823-31. PMID:10581256
Page seeded by OCA on Thu Feb 21 14:42:42 2008