This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1rl6
From Proteopedia
(New page: 200px<br /><applet load="1rl6" size="450" color="white" frame="true" align="right" spinBox="true" caption="1rl6, resolution 2.0Å" /> '''RIBOSOMAL PROTEIN L6'...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1rl6.gif|left|200px]]<br /><applet load="1rl6" size=" | + | [[Image:1rl6.gif|left|200px]]<br /><applet load="1rl6" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="1rl6, resolution 2.0Å" /> | caption="1rl6, resolution 2.0Å" /> | ||
'''RIBOSOMAL PROTEIN L6'''<br /> | '''RIBOSOMAL PROTEIN L6'''<br /> | ||
==Overview== | ==Overview== | ||
| - | In all cells, protein synthesis is coordinated by the ribosome, a large | + | In all cells, protein synthesis is coordinated by the ribosome, a large ribonucleoprotein particle that is composed of > 50 distinct protein molecules and several large RNA molecules. Here we present the crystal structure of ribosomal protein L6 from the thermophilic bacterium Bacillus stearothermophilus solved at 2.6 A resolution. L6 contains two domains with almost identical folds, implying that it was created by an ancient gene duplication event. The surface of the molecule displays several likely sites of interaction with other components of the ribosome. The RNA binding sites appear to be localized in the C-terminal domain whereas the N-terminal domain contains the potential sites for protein-protein interactions. The domain structure is homologous with several other ribosomal proteins and to a large family of eukaryotic RNA binding proteins. |
==About this Structure== | ==About this Structure== | ||
| - | 1RL6 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Geobacillus_stearothermophilus Geobacillus stearothermophilus]. Full crystallographic information is available from [http:// | + | 1RL6 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Geobacillus_stearothermophilus Geobacillus stearothermophilus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1RL6 OCA]. |
==Reference== | ==Reference== | ||
| Line 14: | Line 14: | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Davies, C.]] | [[Category: Davies, C.]] | ||
| - | [[Category: Golden, B | + | [[Category: Golden, B L.]] |
[[Category: Ramakrishnan, V.]] | [[Category: Ramakrishnan, V.]] | ||
| - | [[Category: White, S | + | [[Category: White, S W.]] |
[[Category: alpha/beta protein]] | [[Category: alpha/beta protein]] | ||
[[Category: gentamicin resistance]] | [[Category: gentamicin resistance]] | ||
| Line 22: | Line 22: | ||
[[Category: rna-binding protein]] | [[Category: rna-binding protein]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 14:52:04 2008'' |
Revision as of 12:52, 21 February 2008
|
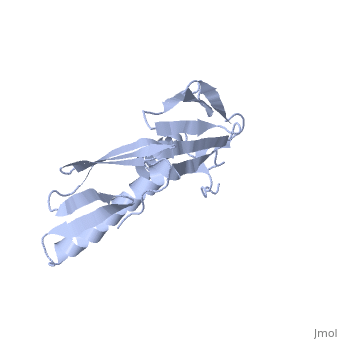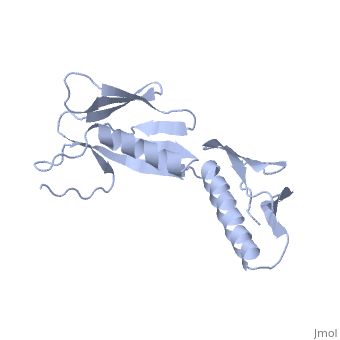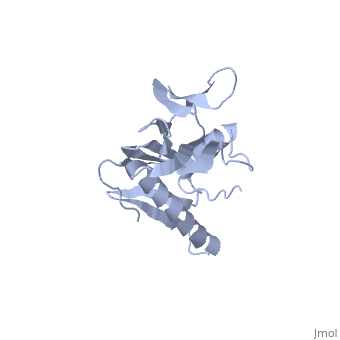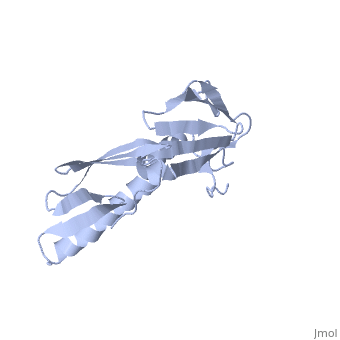
RIBOSOMAL PROTEIN L6
Overview
In all cells, protein synthesis is coordinated by the ribosome, a large ribonucleoprotein particle that is composed of > 50 distinct protein molecules and several large RNA molecules. Here we present the crystal structure of ribosomal protein L6 from the thermophilic bacterium Bacillus stearothermophilus solved at 2.6 A resolution. L6 contains two domains with almost identical folds, implying that it was created by an ancient gene duplication event. The surface of the molecule displays several likely sites of interaction with other components of the ribosome. The RNA binding sites appear to be localized in the C-terminal domain whereas the N-terminal domain contains the potential sites for protein-protein interactions. The domain structure is homologous with several other ribosomal proteins and to a large family of eukaryotic RNA binding proteins.
About this Structure
1RL6 is a Single protein structure of sequence from Geobacillus stearothermophilus. Full crystallographic information is available from OCA.
Reference
Ribosomal protein L6: structural evidence of gene duplication from a primitive RNA binding protein., Golden BL, Ramakrishnan V, White SW, EMBO J. 1993 Dec 15;12(13):4901-8. PMID:8262035
Page seeded by OCA on Thu Feb 21 14:52:04 2008