2oar
From Proteopedia
(→Reference - polishing) |
(Moved theoretical models to a separate Jmol) |
||
| Line 2: | Line 2: | ||
[[Image:2oar.png|left|200px]] | [[Image:2oar.png|left|200px]] | ||
| + | ==Mechanosensitive Channel of Large Conductance (MscL)== | ||
| + | |||
| + | ===Model of Channel Opening=== | ||
| + | <applet load='1kyk' size='300' frame='true' align='right' caption='Theoretical Models' | ||
| + | scene='Goodsell_Sandbox/1kyl/1' /> | ||
| + | Theoretical models<ref>The theoretical models were deposited in the [[PDB]] (before it stopped accepting theoretical models) as [[1kyk]], [[1kyl]], and [[1kym]].</ref> of the <scene name='Goodsell_Sandbox/1kyk/1'>closed, </scene><scene name='Goodsell_Sandbox/1kyl/1'>intermediate,</scene> and <scene name='Goodsell_Sandbox/1kym/1'>open</scene> forms of the channel have been generated based on data from electron paramagnetic resonance spectroscopy, using mutant forms of the protein with spin labels attached to engineered cysteine amino acids in the transmembrane segments<ref>Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Perozo, E, Cortes DM, Sompornpisut, P, Kloda, A, Martinac, B. Nature 2002, 418, 942-948. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/12198539 12198539]</ref>. | ||
| + | |||
| + | {{Clear}} | ||
| + | ---- | ||
| + | <br> | ||
<!-- | <!-- | ||
| - | The line below this paragraph, containing "STRUCTURE_2oar", creates the "Structure Box" on the page. | + | The FIRST line below this paragraph, containing "STRUCTURE_2oar", creates the "Structure Box" on the page. |
You may change the PDB parameter (which sets the PDB file loaded into the applet) | You may change the PDB parameter (which sets the PDB file loaded into the applet) | ||
or the SCENE parameter (which sets the initial scene displayed when the page is loaded), | or the SCENE parameter (which sets the initial scene displayed when the page is loaded), | ||
or leave the SCENE parameter empty for the default display. | or leave the SCENE parameter empty for the default display. | ||
| - | --> | ||
| - | {{STRUCTURE_2oar| PDB=2oar | SCENE= }} | ||
| - | |||
| - | ===Mechanosensitive Channel of Large Conductance (MscL)=== | ||
| - | |||
| - | ==Model of Channel Opening== | ||
| - | Theoretical models<ref>The theoretical models were deposited in the [[PDB]] (before it stopped accepting theoretical models) as [[1kyk]], [[1kyl]], and [[1kym]].</ref> the <scene name='Goodsell_Sandbox/1kyk/1'>closed, </scene><scene name='Goodsell_Sandbox/1kyl/1'>intermediate,</scene> and <scene name='Goodsell_Sandbox/1kym/1'>open</scene> forms of the channel have been generated based on data from electron paramagnetic resonance spectroscopy, using mutant forms of the protein with spin labels attached to engineered cysteine amino acids in the transmembrane segments<ref>Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Perozo, E, Cortes DM, Sompornpisut, P, Kloda, A, Martinac, B. Nature 2002, 418, 942-948. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/12198539 12198539]</ref>. | ||
| - | + | The SECOND line below this paragraph, {{ABSTRACT_PUBMED_9856938}}, adds the Publication Abstract to the page | |
| - | The line below this paragraph, {{ABSTRACT_PUBMED_9856938}}, adds the Publication Abstract to the page | + | |
(as it appears on PubMed at http://www.pubmed.gov), where 9856938 is the PubMed ID number. | (as it appears on PubMed at http://www.pubmed.gov), where 9856938 is the PubMed ID number. | ||
--> | --> | ||
| + | {{STRUCTURE_2oar| PDB=2oar | SCENE= }} | ||
{{ABSTRACT_PUBMED_9856938}} | {{ABSTRACT_PUBMED_9856938}} | ||
Revision as of 22:14, 21 November 2008
Contents |
Mechanosensitive Channel of Large Conductance (MscL)
Model of Channel Opening
|
Theoretical models[1] of the and forms of the channel have been generated based on data from electron paramagnetic resonance spectroscopy, using mutant forms of the protein with spin labels attached to engineered cysteine amino acids in the transmembrane segments[2].
| |||||||
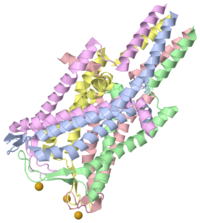
| 2oar, resolution 3.50Å () | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Gene: | mscL (MYCTA) | ||||||
| |||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Mechanosensitive ion channels play a critical role in transducing physical stresses at the cell membrane into an electrochemical response. The MscL family of large-conductance mechanosensitive channels is widely distributed among prokaryotes and may participate in the regulation of osmotic pressure changes within the cell. In an effort to better understand the structural basis for the function of these channels, the structure of the MscL homolog from Mycobacterium tuberculosis was determined by x-ray crystallography to 3.5 angstroms resolution. This channel is organized as a homopentamer, with each subunit containing two transmembrane alpha helices and a third cytoplasmic alpha helix. From the extracellular side, a water-filled opening approximately 18 angstroms in diameter leads into a pore lined with hydrophilic residues which narrows at the cytoplasmic side to an occluded hydrophobic apex that may act as the channel gate. This structure may serve as a model for other mechanosensitive channels, as well as the broader class of pentameric ligand-gated ion channels exemplified by the nicotinic acetylcholine receptor.
Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel., Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC, Science. 1998 Dec 18;282(5397):2220-6. PMID:9856938
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
2OAR [3] is a Single protein structure of sequence from Mycobacterium tuberculosis. This structure supersedes the now removed PDB entry 1msl. Full crystallographic information is available from OCA.
References and Notes
- ↑ The theoretical models were deposited in the PDB (before it stopped accepting theoretical models) as 1kyk, 1kyl, and 1kym.
- ↑ Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Perozo, E, Cortes DM, Sompornpisut, P, Kloda, A, Martinac, B. Nature 2002, 418, 942-948. PMID:12198539
- ↑ Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel., Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC, Science. 1998 Dec 18;282(5397):2220-6. PMID:9856938
Page seeded by OCA on Wed Aug 20 12:12:19 2008