User:Yongmin Kim
From Proteopedia
| Line 6: | Line 6: | ||
== Yongmin Kim, Kwangse Lee, Siwoo Kim == | == Yongmin Kim, Kwangse Lee, Siwoo Kim == | ||
| - | == '''Assignment #1''' == | ||
| - | ''' | + | == '''Glutamic Amino Acid Binding Site''' == |
| - | '''Backbone representation in Rainbow Color''' | ||
| - | <scene name='Glu_Binding_Site/Rainbow/5'>Backbone Trace with Ligand</scene> | ||
| - | + | Glutamate is the first substrate to bind glutamate synthetase. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
The interaction of main-chain carbonyl oxygen containing Nitrogen in Glutamine Synthetase, is replaced by one with the side-chain of Glu-325 and Ser-154.[http://www.ncbi.nlm.nih.gov/pubmed/10708854] The amino acid residues Ser-53 and Asn-264 stabilize Glutamic acid in the binding site. | The interaction of main-chain carbonyl oxygen containing Nitrogen in Glutamine Synthetase, is replaced by one with the side-chain of Glu-325 and Ser-154.[http://www.ncbi.nlm.nih.gov/pubmed/10708854] The amino acid residues Ser-53 and Asn-264 stabilize Glutamic acid in the binding site. | ||
Revision as of 05:09, 16 December 2008
We, Yongmin Kim, Kwangse Lee, and Siwoo Kim, are biology major students at University of Maryland Baltimore Counry, We are enrolled in BIO430 (BioChem), we are planning to complete a project on an aspect of the structure and function of prokaryote glutamine synthetase.
|
Yongmin Kim, Kwangse Lee, Siwoo Kim
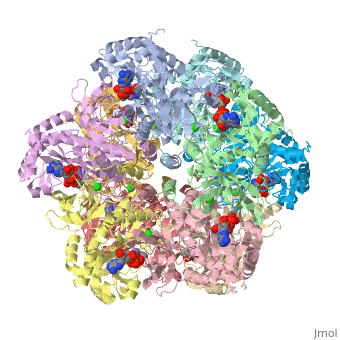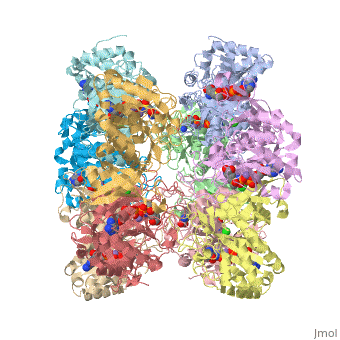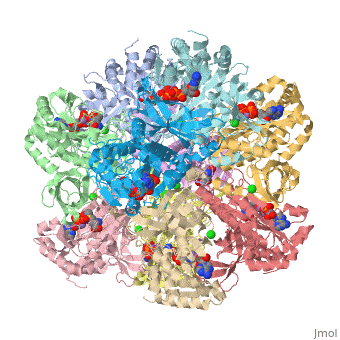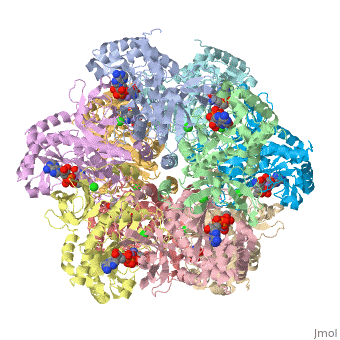
Glutamic Amino Acid Binding Site
Glutamate is the first substrate to bind glutamate synthetase.
The interaction of main-chain carbonyl oxygen containing Nitrogen in Glutamine Synthetase, is replaced by one with the side-chain of Glu-325 and Ser-154.[1] The amino acid residues Ser-53 and Asn-264 stabilize Glutamic acid in the binding site.
Phosphinothricin resides with glutamate substrate pocket and stabilizes the Glu-327 flap in a site which hinders the access of glutamate into the active site, by holding the inhibitor in the enzyme.[2]
When Glutamic amino acid binds in inhibitor Phosphinothricin between Glu-324 ~ 329, only Glu-327 flap which appears when MetSox or Phosphinothricin binds to Glutamine Synthatase will stabilize the orientation of phosphinyl group by guard the glutamate entrance into the bifunnel.[3]
Reference
[1] Krajewski, W. W., et.al.,Structure of Mycobacterium tuberculosis glutamine synthetase in complex with a transition-state mimic provides functional insights, Proc Natl Acad Sci 2005, 102; 10499-10504.
[2] Gill, H & Eisenberg, D., Biochemistry 2001 40: 1903-1912
[3] Eisenberg, D., et.al., Structure-function relationships of glutamine synthetases, Biochim Biophys Acta 2000: 1477, 122-145.