User:Kay Owosela
From Proteopedia
(→Stoichiometry) |
(→Stoichiometry) |
||
| Line 29: | Line 29: | ||
<b><u>Quarternary Interactions</u></b> | <b><u>Quarternary Interactions</u></b> | ||
| - | Interacting subunits are connected through couple of different type of interactions, such as: Salt Bridges, Disulphide bonds, Hydrogen Bonds, and Non-bonded contacts. Each subunit has area’s that are proportional to the surface area of the corresponding protein chains. | + | Interacting subunits are connected through couple of different type of interactions, such as: Salt Bridges, Disulphide bonds, Hydrogen Bonds, and Non-bonded contacts. Each subunit has area’s that are proportional to the surface area of the corresponding protein chains. Each subunit only interacts with four other protomers in the protein. |
= Active Site = | = Active Site = | ||
Revision as of 22:50, 18 December 2008
Biol 430
Biology Major
Partners: Delo Sarr, Mustafa Husain
|
Contents |
Jmol practice
Backbone with ligand
Labeled Ligands in Chain A
Active Site with Amino Acid Residues
Connection between Chain F residue 63 and Chain G residue 319
Stoichiometry
Quarternary Structure
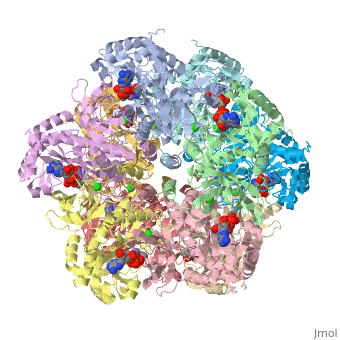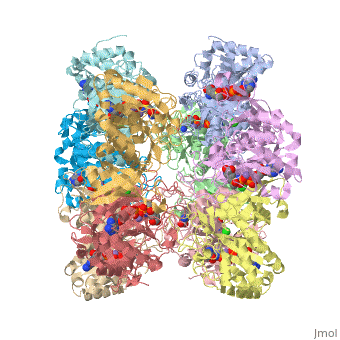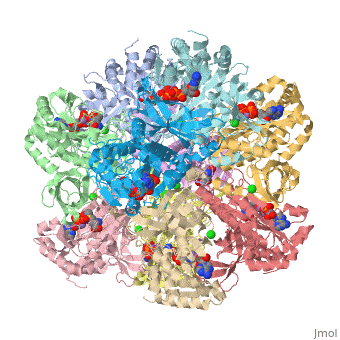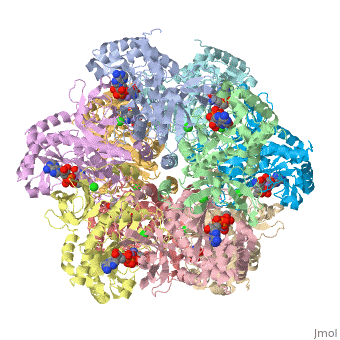
The enzyme Glutamine Synthetase is made up of twelve subunits. The structure consists of two identical hexameric rings held together by hydrophobic and hydrogen bonding forces. The N-terminal helix of each subunit is exposed to hydrophilic solvent, where as the C-terminal is concealed in hydrophobic regions inside the helical structure of the rings. The central channel is linked together by six four-stranded anti-parallel L-sheets which along with the twelve monomers enhance the stability of the ring structure.
Quarternary Interactions
Interacting subunits are connected through couple of different type of interactions, such as: Salt Bridges, Disulphide bonds, Hydrogen Bonds, and Non-bonded contacts. Each subunit has area’s that are proportional to the surface area of the corresponding protein chains. Each subunit only interacts with four other protomers in the protein.
Active Site
In this enzyme, there are 12 similar subunits bonded to each other. Each subunit has an . The active site of glutamine synthetase lies primarily around 3 manganese atoms. It is surrounded by 3 important ligands: MPD, ADP, TL as labeled in the applet. ADP is short for Adenosite Diphosphate. The ligand MPD is (4s)-2-methyl-2,4-pentanediol. And the metal ion, Thallium is also labeled in the applet.
Glutamine Synthetase Symmetry
Reference
1. Eisenberg, David and Gill, Harindarpal and Pfluegl, Gaston and Rotstein, Sergio. “Structure-function relationships of glutamine synthetases.” Elsevier, archives of biochemistry and biophysics 1477(Dec 1999):122-123.
2. Insert Here
3. Insert Here