This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Grace Natalie
From Proteopedia
| Line 21: | Line 21: | ||
γ-carboxylate group of glutatmate, yielding the intermediate <ref name="Eisenberg">D. Eisneberg et al / Biochimica et Biophysica Acta 1477 (2000) 124</ref> . The second step is the attack on the intermediate by ammonia | γ-carboxylate group of glutatmate, yielding the intermediate <ref name="Eisenberg">D. Eisneberg et al / Biochimica et Biophysica Acta 1477 (2000) 124</ref> . The second step is the attack on the intermediate by ammonia | ||
therefore releasing free phosphate to yield glutamine.</font> | therefore releasing free phosphate to yield glutamine.</font> | ||
| - | + | <br> | |
<font size=4 face ="Arial">ATP binding site</font> | <font size=4 face ="Arial">ATP binding site</font> | ||
<br> | <br> | ||
| Line 33: | Line 33: | ||
<br> | <br> | ||
<font size=2>Most residues involved in enzymatic catalysis are located at the C domain but Asp50 is | <font size=2>Most residues involved in enzymatic catalysis are located at the C domain but Asp50 is | ||
| - | contributed from the N domain of the other subunit <scene name='User:Grace_Natalie/Involving_residues_at_one_site/1'>(Involving | + | contributed from the N domain of the other subunit <scene name='User:Grace_Natalie/Involving_residues_at_one_site/1'>(Involving residues at one site of GS)</scene>. |
The binding of ADP induces Asp50 in order to enhance the ammonium binding, and then to deprotonate | The binding of ADP induces Asp50 in order to enhance the ammonium binding, and then to deprotonate | ||
the ammonium ion to form the active ammonia to attack the gamma-glutamyl phosphate.</font> | the ammonium ion to form the active ammonia to attack the gamma-glutamyl phosphate.</font> | ||
| Line 65: | Line 65: | ||
</tr> | </tr> | ||
</table> | </table> | ||
| - | <br> | ||
| - | <scene name='User:Grace_Natalie/Involving_residues/2'>Mapping of Involving Residues</scene> | ||
<br> | <br> | ||
<scene name='User:Grace_Natalie/Gln_synthetase_showing_asp50/1'>Glutamine Synthetase showing ASP50 residue in ATP binding</scene> | <scene name='User:Grace_Natalie/Gln_synthetase_showing_asp50/1'>Glutamine Synthetase showing ASP50 residue in ATP binding</scene> | ||
Revision as of 06:37, 22 December 2008
Background
|
Glutamine synthetase (GS) catalyzes the ATP-dependent condensation of ammonia and
glutamate to yield glutamine, ADP, and inorganic phosphate in the presence of divalent cations
[1] .
The reaction occurs in two steps with γ-glutamyl phosphate as an intermediate and is used by
bacteria to introduce reduced nitrogen into cellular metabolism. GS is a dodecamer formed from
two face-to-face hexameric rings of subunits, with 12 active sites formed between monomers
[2] .
Overall Mechanism
The first step is the formation of the activated intermediate γ-glutamyl phosphate.
The n2 ion coordinates the phosphate oxygens of ATP to allow phosphoryl transfer to the
γ-carboxylate group of glutatmate, yielding the intermediate [3] . The second step is the attack on the intermediate by ammonia
therefore releasing free phosphate to yield glutamine.
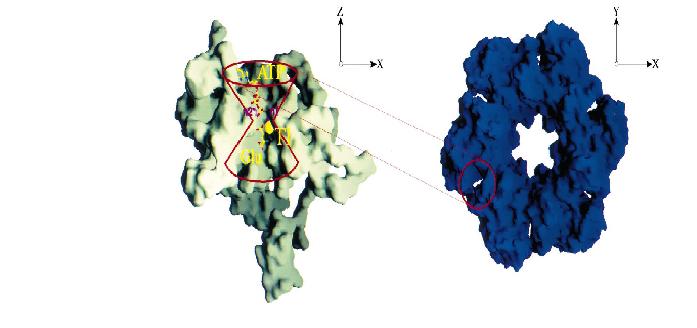
ATP binding site
Each active site of GS is described as a 'bifunnel in which ATP and glutamate bind at opposite ends.
The is referred to as the top of
the bifunnel because it opens to the external 6-fold surface of GS (figure below) [3].
At the the joint of the bifunnel are two cation binding sites, n1 and n2, where either magnesium or manganese bind
for catalysis. The n2 ion is involved in the phosphroyl transfer, while the n1 ion stabilizes an active GS and plays a role in binding glutamate [3] .
Involving Residues
Most residues involved in enzymatic catalysis are located at the C domain but Asp50 is
contributed from the N domain of the other subunit .
The binding of ADP induces Asp50 in order to enhance the ammonium binding, and then to deprotonate
the ammonium ion to form the active ammonia to attack the gamma-glutamyl phosphate.
D. Eisneberg et al / Biochimica et Biophysica Acta 1477 (2000) 124
| Residue | Role in enzymatic mechanism |
| Arg-321 | Coordinates the carboxylate of glutamate |
| Glu-327 | Closes active site and shields intermediate from hydrolysis |
| His-269 | Coordinates the n2 ion |
| Glu-220 | Coordinates the n1 ion |
| Asp-50 | Increases the affinity for ammonium binding |
References
- ↑ 1.0 1.1 Liaw, S-H, et.al.,Discovery of the ammonium substrate site on glutamine synthetase, a third cation binding site Protein Sci. 1995 4: 2358-2365
- ↑ Gill, H & Eisenberg, D., Biochemistry 2001 40: 1903-1912
- ↑ 3.0 3.1 3.2 D. Eisneberg et al / Biochimica et Biophysica Acta 1477 (2000) 124
