1xip
From Proteopedia
(New page: 200px<br /><applet load="1xip" size="450" color="white" frame="true" align="right" spinBox="true" caption="1xip, resolution 2.50Å" /> '''Crystal Structure of...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:1xip.jpg|left|200px]]<br /><applet load="1xip" size=" | + | [[Image:1xip.jpg|left|200px]]<br /><applet load="1xip" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="1xip, resolution 2.50Å" /> | caption="1xip, resolution 2.50Å" /> | ||
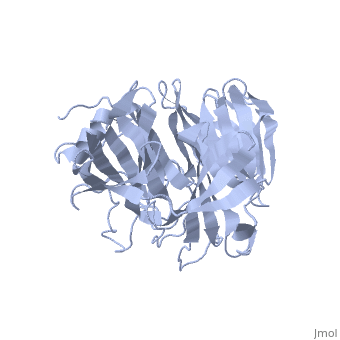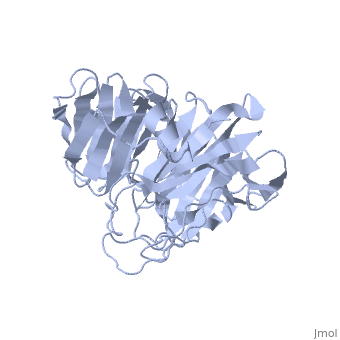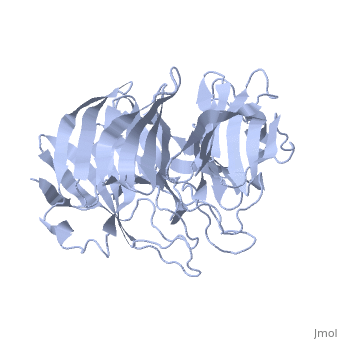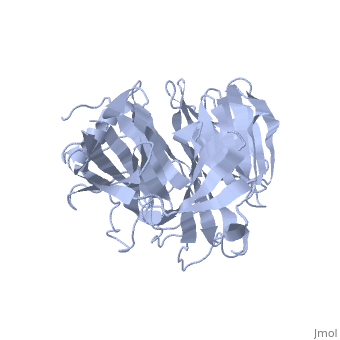
'''Crystal Structure of the N-terminal Domain of Nup159'''<br /> | '''Crystal Structure of the N-terminal Domain of Nup159'''<br /> | ||
==Overview== | ==Overview== | ||
| - | Nuclear export of mRNA in eukaryotic cells is mediated by soluble | + | Nuclear export of mRNA in eukaryotic cells is mediated by soluble transport factors and components of the nuclear pore complex (NPC). The cytoplasmically oriented nuclear pore protein Nup159 plays a critical role in mRNA export through its conserved N-terminal domain (NTD). Here, we report the crystal structure of the Nup159 NTD, refined to 2.5 A. The structure reveals an unusually asymmetric seven-bladed beta-propeller that is structurally conserved throughout eukarya. Using structure-based conservation analysis, we have targeted specific surface residues for mutagenesis. Residue substitutions in a conserved loop of the NTD abolish in vitro binding to Dbp5, a DEAD box helicase required for mRNA export. In vivo, these mutations cause Dbp5 mislocalization and block mRNA export. These findings suggest that the Nup159 NTD functions in mRNA export as a binding platform, tethering shuttling Dbp5 molecules at the nuclear periphery and locally concentrating this mRNA remodeling factor at the cytoplasmic face of the NPC. |
==About this Structure== | ==About this Structure== | ||
| - | 1XIP is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae]. Full crystallographic information is available from [http:// | + | 1XIP is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1XIP OCA]. |
==Reference== | ==Reference== | ||
| Line 13: | Line 13: | ||
[[Category: Saccharomyces cerevisiae]] | [[Category: Saccharomyces cerevisiae]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| - | [[Category: Berger, J | + | [[Category: Berger, J M.]] |
| - | [[Category: Erzberger, J | + | [[Category: Erzberger, J P.]] |
| - | [[Category: Weirich, C | + | [[Category: Weirich, C S.]] |
[[Category: Weis, K.]] | [[Category: Weis, K.]] | ||
[[Category: beta-propeller]] | [[Category: beta-propeller]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 15:55:15 2008'' |
Revision as of 13:55, 21 February 2008
|
Crystal Structure of the N-terminal Domain of Nup159
Overview
Nuclear export of mRNA in eukaryotic cells is mediated by soluble transport factors and components of the nuclear pore complex (NPC). The cytoplasmically oriented nuclear pore protein Nup159 plays a critical role in mRNA export through its conserved N-terminal domain (NTD). Here, we report the crystal structure of the Nup159 NTD, refined to 2.5 A. The structure reveals an unusually asymmetric seven-bladed beta-propeller that is structurally conserved throughout eukarya. Using structure-based conservation analysis, we have targeted specific surface residues for mutagenesis. Residue substitutions in a conserved loop of the NTD abolish in vitro binding to Dbp5, a DEAD box helicase required for mRNA export. In vivo, these mutations cause Dbp5 mislocalization and block mRNA export. These findings suggest that the Nup159 NTD functions in mRNA export as a binding platform, tethering shuttling Dbp5 molecules at the nuclear periphery and locally concentrating this mRNA remodeling factor at the cytoplasmic face of the NPC.
About this Structure
1XIP is a Single protein structure of sequence from Saccharomyces cerevisiae. Full crystallographic information is available from OCA.
Reference
The N-terminal domain of Nup159 forms a beta-propeller that functions in mRNA export by tethering the helicase Dbp5 to the nuclear pore., Weirich CS, Erzberger JP, Berger JM, Weis K, Mol Cell. 2004 Dec 3;16(5):749-60. PMID:15574330
Page seeded by OCA on Thu Feb 21 15:55:15 2008