2af5
From Proteopedia
(New page: 200px<br /><applet load="2af5" size="450" color="white" frame="true" align="right" spinBox="true" caption="2af5, resolution 2.50Å" /> '''2.5A X-ray Structure...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:2af5.gif|left|200px]]<br /><applet load="2af5" size=" | + | [[Image:2af5.gif|left|200px]]<br /><applet load="2af5" size="350" color="white" frame="true" align="right" spinBox="true" |
caption="2af5, resolution 2.50Å" /> | caption="2af5, resolution 2.50Å" /> | ||
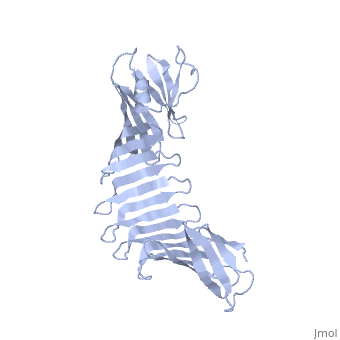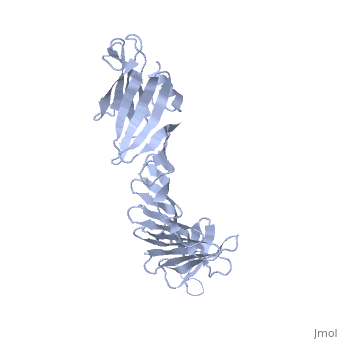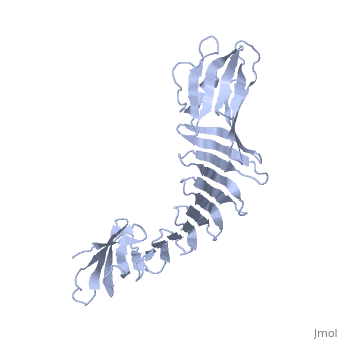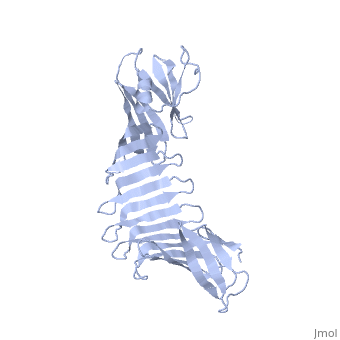
'''2.5A X-ray Structure of Engineered OspA protein'''<br /> | '''2.5A X-ray Structure of Engineered OspA protein'''<br /> | ||
==Overview== | ==Overview== | ||
| - | Although the beta-rich self-assemblies are a major structural class for | + | Although the beta-rich self-assemblies are a major structural class for polypeptides and the focus of intense research, little is known about their atomic structures and dynamics due to their insoluble and noncrystalline nature. We developed a protein engineering strategy that captures a self-assembly segment in a water-soluble molecule. A predefined number of self-assembling peptide units are linked, and the beta-sheet ends are capped to prevent aggregation, which yields a mono-dispersed soluble protein. We tested this strategy by using Borrelia outer surface protein (OspA) whose single-layer beta-sheet located between two globular domains consists of two beta-hairpin units and thus can be considered as a prototype of self-assembly. We constructed self-assembly mimics of different sizes and determined their atomic structures using x-ray crystallography and NMR spectroscopy. Highly regular beta-sheet geometries were maintained in these structures, and peptide units had a nearly identical conformation, supporting the concept that a peptide in the regular beta-geometry is primed for self-assembly. However, we found small but significant differences in the relative orientation between adjacent peptide units in terms of beta-sheet twist and bend, suggesting their inherent flexibility. Modeling shows how this conformational diversity, when propagated over a large number of peptide units, can lead to a substantial degree of nanoscale polymorphism of self-assemblies. |
==About this Structure== | ==About this Structure== | ||
| - | 2AF5 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Borrelia_burgdorferi Borrelia burgdorferi]. Full crystallographic information is available from [http:// | + | 2AF5 is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Borrelia_burgdorferi Borrelia burgdorferi]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2AF5 OCA]. |
==Reference== | ==Reference== | ||
| Line 23: | Line 23: | ||
[[Category: single-layer beta-sheet]] | [[Category: single-layer beta-sheet]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 16:26:49 2008'' |
Revision as of 14:26, 21 February 2008
|
2.5A X-ray Structure of Engineered OspA protein
Overview
Although the beta-rich self-assemblies are a major structural class for polypeptides and the focus of intense research, little is known about their atomic structures and dynamics due to their insoluble and noncrystalline nature. We developed a protein engineering strategy that captures a self-assembly segment in a water-soluble molecule. A predefined number of self-assembling peptide units are linked, and the beta-sheet ends are capped to prevent aggregation, which yields a mono-dispersed soluble protein. We tested this strategy by using Borrelia outer surface protein (OspA) whose single-layer beta-sheet located between two globular domains consists of two beta-hairpin units and thus can be considered as a prototype of self-assembly. We constructed self-assembly mimics of different sizes and determined their atomic structures using x-ray crystallography and NMR spectroscopy. Highly regular beta-sheet geometries were maintained in these structures, and peptide units had a nearly identical conformation, supporting the concept that a peptide in the regular beta-geometry is primed for self-assembly. However, we found small but significant differences in the relative orientation between adjacent peptide units in terms of beta-sheet twist and bend, suggesting their inherent flexibility. Modeling shows how this conformational diversity, when propagated over a large number of peptide units, can lead to a substantial degree of nanoscale polymorphism of self-assemblies.
About this Structure
2AF5 is a Single protein structure of sequence from Borrelia burgdorferi. Full crystallographic information is available from OCA.
Reference
Atomic structures of peptide self-assembly mimics., Makabe K, McElheny D, Tereshko V, Hilyard A, Gawlak G, Yan S, Koide A, Koide S, Proc Natl Acad Sci U S A. 2006 Nov 21;103(47):17753-8. Epub 2006 Nov 8. PMID:17093048
Page seeded by OCA on Thu Feb 21 16:26:49 2008