Factor IX
From Proteopedia
| Line 45: | Line 45: | ||
[[Image:Research_02_big4.JPG|frame|right|http://www.molecular-haemostasis.de/grfx/pix/]] | [[Image:Research_02_big4.JPG|frame|right|http://www.molecular-haemostasis.de/grfx/pix/]] | ||
The post-translational modification of the glutamic acid residues of the FIX polypeptide is carried out by vitamin K-dependent ''γ''-glutamyl carboxylase (1). Vitamin K-dependent carboxylase is a membrane associated protein in the endoplasmic reticulum. It converts a multiple of glutamic acid residues which are located within 40 residues of a propeptide-containing sequence into ''γ''-carboxyglutamate (2). | The post-translational modification of the glutamic acid residues of the FIX polypeptide is carried out by vitamin K-dependent ''γ''-glutamyl carboxylase (1). Vitamin K-dependent carboxylase is a membrane associated protein in the endoplasmic reticulum. It converts a multiple of glutamic acid residues which are located within 40 residues of a propeptide-containing sequence into ''γ''-carboxyglutamate (2). | ||
| + | |||
| + | The association ofvitamin K-dependent carboxylase and its substrates is dependent on the 18-amino acid propeptide sequence (3, 4). The propeptide anchors the substrate to the carboxylase for a carboxylation reaction to occur. This binding of the propeptide to carboxylase stimulates the incorporation of carbon dioxide into the glutamate residues with the use of a reduced vitamin K and oxygen (4, 5). The residues 495-513 of the carboxylase function as an internal propeptide that is homologous to the the propeptide sequence. | ||
| + | |||
| + | To identify the internal propetide region of the Vitamin K-dependent carboxylase, five mutant carboxylase molecules (F496A, V502A, Q503R, Q503N, and P504Q) were generated identify the residues responsible for homologous substrate propeptide binding. | ||
Revision as of 18:39, 26 April 2009
-----This page is still under construction-------
|
Factor IX (plasma thromboplastin component, Christmas factor, or hemophilia B factor) is a 57-kDa vitamin K-dependent procoagulant glycoprotein. It is synthesized by the liver hepatocyte as a pre-prozymogen that requires extensive posttranslational modification. The pre-prozymogen contains a pre-peptide (hydrophobic signal peptide) at its amino terminal that transports the growing polypeptide into the lumen of the Endoplasmic Reticulum. Once inside the ER, this signal peptide is cleaved by a signal peptidase. A pro-peptide functions as a recognition element for a vitamin K-dependent carboxylase (γ-glutamyl carboxylase) which modifies 12 glutamic acid residues to gamma-carboxyglutamyl ( ) residues. These residues are required for the association with the anionic phospholipid surface through Ca2+-dependent binding. The Domain is followed by two epidermal growth factor domains ( and ). The N-terminus of contains a Ca2+ binding site, while the C-terminus connects to a hydrophobic pocket of and a salt bridge through Lys122 ( residue) and Gln74 (). connects to the domain through a linker peptide and is required for a proper orientation and folding of . To have a physiologically active factor IX, two cleaves must occur to remove a 35 amino acid region that precedes the catalytic region. The first cleave is at Arg145, generating an inactive FIXα. The second cleavage is at Arg180 results in a catalytically active molecule FIXaβ. This resulting heterodimer is held by a disulfide bridge at Cys132-Cys289. The contains a catalytic triad of . Upon cleave at Arg180, Val181 can form a salt bridge with Asp364, which is a characteristic of active . The active FIXa, can then interact with its cofactor, FVIIIa, to form a membrane-bound Xase complex, which activated FX to FXa.
Gene Structure and Expression:
The gene for factor IX is located on the long arm of chromosome X between positions 26.3- and 27.1 and contains eight exons and seven introns, which segregate the FIX gene into specific structural regions.
| I | II | III | IV | V | VI | VII | VIII |
Exon I encodes the hydrophobic signal peptide that targets the FIX into the lumen of the Endoplasmic Reticulum. Exon II codes for the pro-peptide and the Gla domain. Exon III encodes the , that inserts itself into the lipid membrane, anchoring FIX. Exon IV and V, code for EGF-1 and EGF-2, the serine protease region is encoded by exon VI-VIII.
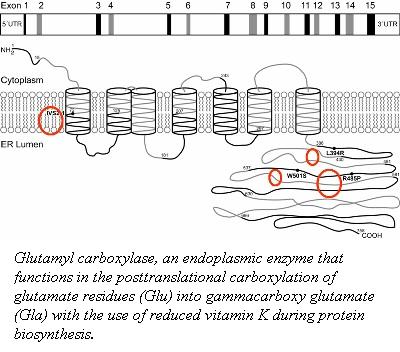
γ-Carboxyglutamate: Post-translational carboxylation of glutamate residues (Glu) into gammacarboxy glutamate (Gla)
The post-translational modification of the glutamic acid residues of the FIX polypeptide is carried out by vitamin K-dependent γ-glutamyl carboxylase (1). Vitamin K-dependent carboxylase is a membrane associated protein in the endoplasmic reticulum. It converts a multiple of glutamic acid residues which are located within 40 residues of a propeptide-containing sequence into γ-carboxyglutamate (2).
The association ofvitamin K-dependent carboxylase and its substrates is dependent on the 18-amino acid propeptide sequence (3, 4). The propeptide anchors the substrate to the carboxylase for a carboxylation reaction to occur. This binding of the propeptide to carboxylase stimulates the incorporation of carbon dioxide into the glutamate residues with the use of a reduced vitamin K and oxygen (4, 5). The residues 495-513 of the carboxylase function as an internal propeptide that is homologous to the the propeptide sequence.
To identify the internal propetide region of the Vitamin K-dependent carboxylase, five mutant carboxylase molecules (F496A, V502A, Q503R, Q503N, and P504Q) were generated identify the residues responsible for homologous substrate propeptide binding.
|
|
Proteopedia Page Contributors and Editors (what is this?)
Nadia Dorochko, Michal Harel, Alexander Berchansky, David Canner