AChE inhibitors and substrates (Part II)
From Proteopedia
| Line 23: | Line 23: | ||
{{Clear}} | {{Clear}} | ||
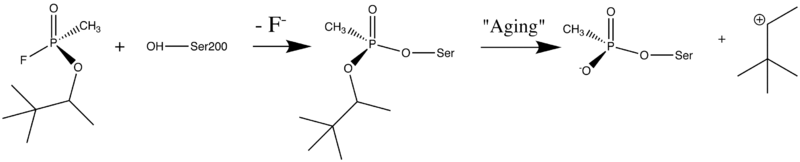
| - | <applet load='AC1.pdb' size='500' frame='true' align=' | + | '''9)''' Organophosphorus acid anhydride (OP) nerve agents are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated. |
| + | |||
| + | [[Image:Soman_reaction.png | left | thumb | 800px | Reaction between Ser200OG and Soman, assuming an in-line attack by the OG, followed by spontaneous dealkylation of the O-pinacolyl group.]] | ||
| + | <br style="clear:both;"/> | ||
| + | <br /><applet load="1som" size="350" color="white" frame="true" align="right" spinBox="true" | ||
| + | caption="1som, resolution 2.20Å" scene="1som/Ache_soman/1"/> <br /> | ||
| + | To understand the basis for irreversible inhibition, we solved the structure of the aged conjugate obtained by reaction of ''Torpedo californica'' AChE (''Tc''AChE) with [http://en.wikipedia.org/wiki/Soman O-pinacolylmethylphosphonofluoridate] (soman) by X-ray crystallography to 2.2Å resolution. The highest positive difference density peak corresponded to the OP phosphorus and was located within covalent bonding distance of the active-site serine (S200). The <scene name='1som/Soman_active_site/3'>OP-oxygen atoms</scene> are within hydrogen-bonding distance of four potential donors from catalytic subsites of the enzyme, suggesting that electrostatic forces significantly stabilize the aged enzyme. The methyl group of soman occupies the <scene name='1som/Soman_acyl_binding/2'>acyl binding pocket</scene>, bounded by Trp233, Phe288, and Phe290. The active sites of aged sarin-TcAChE ([[1cfj]]) and soman-TcAChE were essentially identical and provided structural models for the negatively charged, tetrahedral intermediate that occurs during deacylation with the natural substrate, acetylcholine. | ||
| + | |||
| + | <applet load='AC1.pdb' size='500' frame='true' align='left' | ||
scene='2vja/Common/1' /> | scene='2vja/Common/1' /> | ||
| - | ''' | + | '''10)''' <scene name='2vja/Common/3'>OTMA</scene> is a nonhydrolyzable substrate analogue of AChE. Its hydrolysis is impossible as OTMA possesses <scene name='2vja/Common/4'>carbon</scene> atom instead of the <scene name='2vja/Common/5'>ester oxygen</scene> in the AChE natural substrate ACh. Similarly to ACh, OTMA covalently binds to the ''Tc''AChE ([[2vja]]) <scene name='2vja/Active_site/1'>Ser200</scene> Oγ at the CAS. At this subsite OTMA also interacts with <scene name='2vja/Active_site/2'>Trp84, Phe330</scene> (cation-π interactions); <scene name='2vja/Active_site/3'>Glu199</scene> (electrostatic interaction); <scene name='2vja/Active_site/4'>Gly118, Gly119, and Ala201</scene>(hydrogen bonds). OTMA binds not only at CAS, but also at PAS. A second OTMA molecule interacts with <scene name='2vja/Active_site/5'>Trp279, Tyr70</scene> (cation-π interactions), and <scene name='2vja/Active_site/6'>Tyr121</scene> (weak hydrogen bond). Thus, this dual binding mode of OTMA with ''Tc''AChE (to CAS and PAS) could be prototypical for [[AChE bivalent inhibitors]]. |
{{Clear}} | {{Clear}} | ||
Revision as of 12:41, 25 June 2009
AChE monovalent inhibitors (continuation of the page AChE inhibitors and substrates)
|
6) Edrophonium (EDR) (2ack) is stacked between the aromatic rings of , near the TcAChE which consists of S200, E327, and H440.
|
7) Rivastigmine (Exelon) is a carbamate inhibitor of AChE, and it is currenly used in therapy of Alzheimer's disease. Rivastigmine (colored yellow) interacts with TcAChE (colored lime) at the . The carbamyl moiety of rivastigmine is to the active-site S200 Oγ. The second part of rivastigmine (the leaving group), NAP ((−)-S-3-[1-(dimethylamino)ethyl]phenol) is also held in the active-site gorge, but it is from the carbamyl moiety, hence, carbamylation took place. The of TcAChE/NAP (colored magenta) is known (1gqs). The TcAChE active-site residues which are interacting with NAP are colored violet. NAP is located in a similar region of TcAChE active site, but with different orientation than that of the NAP part (colored yellow) in the TcAChE/rivastigmine complex. Only H440 and F330 significantly change their side-chain conformations. of the TcAChE active sites in 4 different structures (TcAChE/rivastigmine, TcAChE/NAP, native TcAChE(2ace), and TcAChE/VX (1vxr, TcAChE colored white and VX black)) reveals that the conformation of H440 in the TcAChE/NAP structure is very similar its conformation in the native TcAChE (2ace), but the distance between H440 Nδ and E327 Oε is significantly longer in the TcAChE/rivastigmine and the TcAChE/VX complexes. This structural change disrupts the catalytic triad consisting of S200, E327, H440. This could explain the very slow kinetics of AChE reactivation after its inhibition by rivastigmine.
|
8) The TcAChE active site consists of two binding subsites. One of them is the "catalytic anionic site" (CAS), which involves the catalytic triad (colored orange) and the conserved residues which also participate in ligand recognition. Another conserved residue (colored cyan) is situated at the second binding subsite, termed the "peripheral anionic site" (PAS), ~14 Å from CAS. is a good example of the PAS-binding AChE inhibitors. of the crystal structure of the edrophonium /TcAChE (mentioned above as a CAS-binding inhibitor) (2ack) on the thioflavin T /TcAChE complex structure (2j3q) shows that these ligands' positions do not overlap. Of note is that Phe330, which is part of the CAS, is the single residue interacting with thioflavin T. This residue is the only one which significantly to avoid clashes in comparison to other CAS residues of the edrophonium /TcAChE complex.
9) Organophosphorus acid anhydride (OP) nerve agents are potent inhibitors which rapidly phosphonylate AChE and then may undergo an internal dealkylation reaction (called "aging") to produce an OP-enzyme conjugate that cannot be reactivated.
|
To understand the basis for irreversible inhibition, we solved the structure of the aged conjugate obtained by reaction of Torpedo californica AChE (TcAChE) with O-pinacolylmethylphosphonofluoridate (soman) by X-ray crystallography to 2.2Å resolution. The highest positive difference density peak corresponded to the OP phosphorus and was located within covalent bonding distance of the active-site serine (S200). The are within hydrogen-bonding distance of four potential donors from catalytic subsites of the enzyme, suggesting that electrostatic forces significantly stabilize the aged enzyme. The methyl group of soman occupies the , bounded by Trp233, Phe288, and Phe290. The active sites of aged sarin-TcAChE (1cfj) and soman-TcAChE were essentially identical and provided structural models for the negatively charged, tetrahedral intermediate that occurs during deacylation with the natural substrate, acetylcholine.
|
10) is a nonhydrolyzable substrate analogue of AChE. Its hydrolysis is impossible as OTMA possesses atom instead of the in the AChE natural substrate ACh. Similarly to ACh, OTMA covalently binds to the TcAChE (2vja) Oγ at the CAS. At this subsite OTMA also interacts with (cation-π interactions); (electrostatic interaction); (hydrogen bonds). OTMA binds not only at CAS, but also at PAS. A second OTMA molecule interacts with (cation-π interactions), and (weak hydrogen bond). Thus, this dual binding mode of OTMA with TcAChE (to CAS and PAS) could be prototypical for AChE bivalent inhibitors.
Please see also our pages AChE bivalent inhibitors and AChE bivalent inhibitors (Part II).
Selected 3D Structures of AChE
- 2ace This is the original solved structure for Torpedo Californica
- 1ea5 This is one of the highest quality representative X-ray structures in the PDB.
- 1eve The E2020 (Aricept) complex.
- 1ax9 Endrophonium complex.
- 1vot Complex with Huperzine, a Chinese folk medicine.
- 1fss Complex with snake venum toxin Fasciculin-II.
- 1acj Complex with tacrine.
- 1e66 Complex with huprine X.
- 1dx6 Complex with galanthamine.
- 1w6r Complex with galanthamine iminium derivative.
- 2ack Complex with edrophonium.
- 1vzj Structure of the tetramerization domain of acetylcholinesterase.
- 1gqr Complex with rivastigmine.
- 1gqs Complex with NAP alone.
- 1vxr Complex with VX.
- 2vja Complex with OTMA.
References
- Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol. 1997 Jan;4(1):57-63. PMID:8989325
- Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9031-5. PMID:8415649
- Greenblatt HM, Guillou C, Guenard D, Argaman A, Botti S, Badet B, Thal C, Silman I, Sussman JL. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: implications for structure-based drug design. J Am Chem Soc. 2004 Dec 1;126(47):15405-11. PMID:15563167 doi:http://dx.doi.org/10.1021/ja0466154
- Ravelli RB, Raves ML, Ren Z, Bourgeois D, Roth M, Kroon J, Silman I, Sussman JL. Static Laue diffraction studies on acetylcholinesterase. Acta Crystallogr D Biol Crystallogr. 1998 Nov 1;54(Pt 6 Pt 2):1359-66. PMID:10089512
- Haviv H, Wong DM, Greenblatt HM, Carlier PR, Pang YP, Silman I, Sussman JL. Crystal packing mediates enantioselective ligand recognition at the peripheral site of acetylcholinesterase. J Am Chem Soc. 2005 Aug 10;127(31):11029-36. PMID:16076210 doi:http://dx.doi.org/10.1021/ja051765f
- Carlier PR, Du DM, Han Y, Liu J, Pang YP. Potent, easily synthesized huperzine A-tacrine hybrid acetylcholinesterase inhibitors. Bioorg Med Chem Lett. 1999 Aug 16;9(16):2335-8. PMID:10476864
- Wong DM, Greenblatt HM, Dvir H, Carlier PR, Han YF, Pang YP, Silman I, Sussman JL. Acetylcholinesterase complexed with bivalent ligands related to huperzine a: experimental evidence for species-dependent protein-ligand complementarity. J Am Chem Soc. 2003 Jan 15;125(2):363-73. PMID:12517147 doi:http://dx.doi.org/10.1021/ja021111w
- Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 A resolution. FEBS Lett. 1999 Dec 17;463(3):321-6. PMID:10606746
- Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002 Mar 19;41(11):3555-64. PMID:11888271
- Colletier JP, Bourgeois D, Sanson B, Fournier D, Sussman JL, Silman I, Weik M. Shoot-and-Trap: use of specific x-ray damage to study structural protein dynamics by temperature-controlled cryo-crystallography. Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11742-7. Epub 2008 Aug 13. PMID:18701720
- Dvir H, Wong DM, Harel M, Barril X, Orozco M, Luque FJ, Munoz-Torrero D, Camps P, Rosenberry TL, Silman I, Sussman JL. 3D structure of Torpedo californica acetylcholinesterase complexed with huprine X at 2.1 A resolution: kinetic and molecular dynamic correlates. Biochemistry. 2002 Mar 5;41(9):2970-81. PMID:11863435
- Harel M, Sonoda LK, Silman I, Sussman JL, Rosenberry TL. Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J Am Chem Soc. 2008 Jun 25;130(25):7856-61. Epub 2008 May 31. PMID:18512913 doi:http://dx.doi.org/10.1021/ja7109822
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Wayne Decatur, David Canner, Michal Harel