Green Fluorescent Protein
From Proteopedia
(→Notes and Literature References) |
|||
| Line 20: | Line 20: | ||
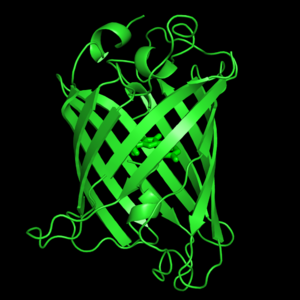
<scene name='Green_Fluorescent_Protein/1ema_gfp_defaultview/2'>GFP resembles a barrel.</scene><br> | <scene name='Green_Fluorescent_Protein/1ema_gfp_defaultview/2'>GFP resembles a barrel.</scene><br> | ||
| - | The crystal structure of GFP <ref> | + | The crystal structure of GFP <ref>PMID:8703075</ref><ref>PMID:9631087</ref> is an eleven-stranded anti-parallel beta-barrel, threaded by an alpha-helix, running up along the axis of the cylinder. In <scene name='Green_Fluorescent_Protein/1ema_gfp_barrel/1'>this view</scene> the structure is colored by secondary structure({{Template:ColorKey_Helix}} and {{Template:ColorKey_Strand}}) to better show the eleven strands and the helices. |
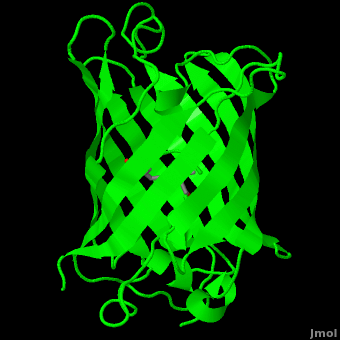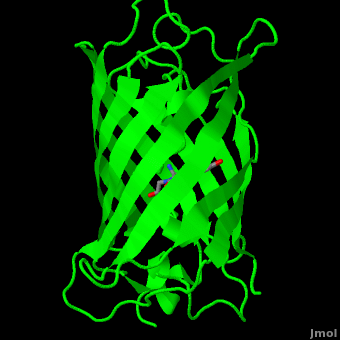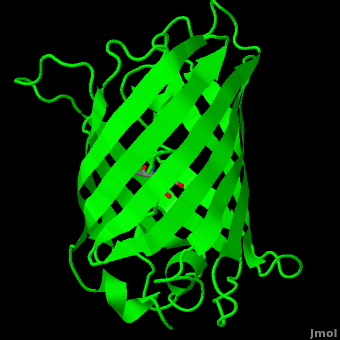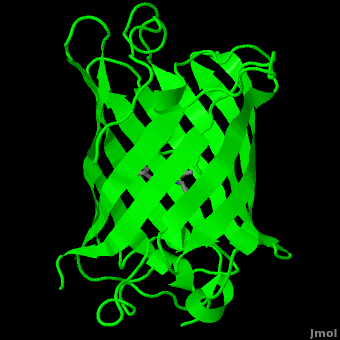
<scene name='Green_Fluorescent_Protein/1ema_gfp_chromophorezoom/5'>The chromophore</scene>:<br> | <scene name='Green_Fluorescent_Protein/1ema_gfp_chromophorezoom/5'>The chromophore</scene>:<br> | ||
The chromophore is in the distorted alpha-helix that runs along the axis of the can, close to the center of the can-like cylinder. <br> | The chromophore is in the distorted alpha-helix that runs along the axis of the can, close to the center of the can-like cylinder. <br> | ||
| - | The chromophore is formed from the tripeptide motif Serine65-Tyrosine66-Glycine67 after translation and folding of GFP. As the GFP protein folds into its native conformation, these three amino acids are forced into a sharp turn, greatly favoring a nucleophilic attack of the amide of Glycine67 on the carbonyl of Serine65, leading to imidazolinone formation by cyclization and dehydration. At this point, GFP is not fluorescent; however, in the presence of molecular oxygen, the α–β bond of Tyrosine66 is subsequently dehydrogenated into conjugation with the imidazolinone, which results in maturation of the GFP chromophore to its fluorescent form <ref> | + | The chromophore is formed from the tripeptide motif Serine65-Tyrosine66-Glycine67 after translation and folding of GFP. As the GFP protein folds into its native conformation, these three amino acids are forced into a sharp turn, greatly favoring a nucleophilic attack of the amide of Glycine67 on the carbonyl of Serine65, leading to imidazolinone formation by cyclization and dehydration. At this point, GFP is not fluorescent; however, in the presence of molecular oxygen, the α–β bond of Tyrosine66 is subsequently dehydrogenated into conjugation with the imidazolinone, which results in maturation of the GFP chromophore to its fluorescent form <ref>PMID:7809066</ref><ref>PMID:8578587</ref>.<br> |
The chromophore <scene name='Green_Fluorescent_Protein/1ema_chromophorecloseup/2'>close-up view</scene>. | The chromophore <scene name='Green_Fluorescent_Protein/1ema_chromophorecloseup/2'>close-up view</scene>. | ||
| Line 31: | Line 31: | ||
==Reference for the Structure== | ==Reference for the Structure== | ||
| - | + | <ref group="xtra">PMID:8703075</ref><ref group="xtra"> | |
==Related Structures and Topics== | ==Related Structures and Topics== | ||
Revision as of 03:52, 17 October 2009
Green Flourscent Protein
with flourescence-conferring chromophore
at center of the barrel-like structure
Background
Osamu Shimomura, Martin Chalfie and Roger Y. Tsien shared the 2008 Nobel Prize in Chemistry for their for the discovery and development of the green fluorescent protein, GFP.
GFP is small protein (21 kDa) that naturally occurs in the jellyfish Aequorea victoria and does not require cofactors to become fluorescent. The chromophore at the center of the structure that is responsible for its fluorescence is formed
spontaneously from a tri-peptide motif in the primary structure of GFP. Being small and facile has lead to its use by investigators as a tool to examine a multitude of processes in many organisms; often in such research, GFP is fused to other proteins genetically.
Structure
| |||||||
The crystal structure of GFP [1][2] is an eleven-stranded anti-parallel beta-barrel, threaded by an alpha-helix, running up along the axis of the cylinder. In the structure is colored by secondary structure(Alpha Helices and Beta Strands ) to better show the eleven strands and the helices.
:
The chromophore is in the distorted alpha-helix that runs along the axis of the can, close to the center of the can-like cylinder.
The chromophore is formed from the tripeptide motif Serine65-Tyrosine66-Glycine67 after translation and folding of GFP. As the GFP protein folds into its native conformation, these three amino acids are forced into a sharp turn, greatly favoring a nucleophilic attack of the amide of Glycine67 on the carbonyl of Serine65, leading to imidazolinone formation by cyclization and dehydration. At this point, GFP is not fluorescent; however, in the presence of molecular oxygen, the α–β bond of Tyrosine66 is subsequently dehydrogenated into conjugation with the imidazolinone, which results in maturation of the GFP chromophore to its fluorescent form [3][4].
The chromophore .
Reference for the Structure
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Wayne Decatur, Karsten Theis, Eran Hodis, Laura Carbone, Karl Oberholser, Mark Hoelzer, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Marius Mihasan, Joseph M. Steinberger, David Canner