User:Tilman Schirmer/Sandbox 212
From Proteopedia
(Difference between revisions)
(→Activation ''via'' dimerization) |
(→activated state) |
||
| Line 24: | Line 24: | ||
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| - | <scene name='User:Tilman_Schirmer/Sandbox_212/Tight_dimer/3'>Asp 53 modified by BeF3-</scene> | + | <scene name='User:Tilman_Schirmer/Sandbox_212/Tight_dimer/3'>Asp 53 modified by BeF3-</scene> to mimick phosphorylation, <scene name='User:Tilman_Schirmer/Sandbox_212/Tight_dimer/4'>tight dimeric stem</scene> in the crystal |
<br><br> | <br><br> | ||
Revision as of 08:19, 15 July 2009
PleD activation
Activation via dimerization
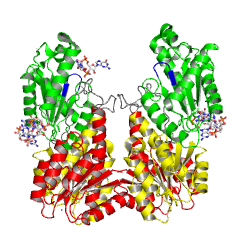
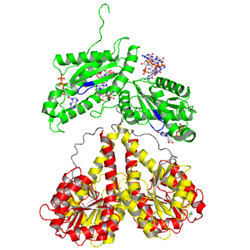
Activation of PleD proceeds via phosphorylation-induced dimerization. Upon modification of Asp53 of the Rec domain, the intramolecular packing of the Rec and Rec' domains is changed. This in turn, reduces the Kd of dimerization considerably, because the dimerization interface of the (Rec-Rec')2 "stem" is improved.
Stem mediated dimerization enhances diguanylate cyclase efficiency, because it favors formation of the catalytically competent (GGDEF)2 dimer (not shown here , but see back, bottom of page).
non-active state
|
monomer in solution, in the crystal
activated state
|
to mimick phosphorylation, in the crystal