User:Tilman Schirmer/Sandbox 211
From Proteopedia
(→Substrate binding) |
|||
| Line 40: | Line 40: | ||
After binding of the <scene name='User:Tilman_Schirmer/Sandbox_211/Competent_ggdef_dimer/6'>GTP substrate</scene> (here we show GMP, <i>please update</i>), it is believed that two GGDEF domains associate antiparallely to form a catalytically competent dimer (<scene name='User:Tilman_Schirmer/Sandbox_211/Competent_ggdef_dimer/3'>view1</scene>, <scene name='User:Tilman_Schirmer/Sandbox_211/Competent_ggdef_dimer/1'>view2</scene>). Then, by inter-molecular nucleophilic in-line attack of the O3' atom onto the α-phosphorous of the other GTP substrate molecule, two phosphodiester bonds are formed under release of two pyrophosphate molecules (2 GTP -> c-di-GMP + 2 PPi). | After binding of the <scene name='User:Tilman_Schirmer/Sandbox_211/Competent_ggdef_dimer/6'>GTP substrate</scene> (here we show GMP, <i>please update</i>), it is believed that two GGDEF domains associate antiparallely to form a catalytically competent dimer (<scene name='User:Tilman_Schirmer/Sandbox_211/Competent_ggdef_dimer/3'>view1</scene>, <scene name='User:Tilman_Schirmer/Sandbox_211/Competent_ggdef_dimer/1'>view2</scene>). Then, by inter-molecular nucleophilic in-line attack of the O3' atom onto the α-phosphorous of the other GTP substrate molecule, two phosphodiester bonds are formed under release of two pyrophosphate molecules (2 GTP -> c-di-GMP + 2 PPi). | ||
| + | |||
| + | ==References== | ||
| + | Activated PleD structure [[2v0n]]: | ||
| + | <ref group="xtra">PMID:17697997</ref> <ref group="xtra">PMID:15075296</ref> <ref group="xtra">PMID:17640875</ref> | ||
| + | <references group="xtra"/> | ||
Revision as of 08:44, 15 July 2009
PleD
Contents |
Overview
|
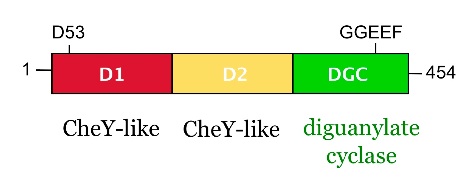
from Caulobacter crescentus is a response regulator with an unorthodox catalytic, diguanylate cyclase, output domain. It is composed of a canonical CheY-like response regulator receiver (, also called D1) domain,
a Rec-like (, also called D2) adaptor domain,
and a C-terminal domain that confers the catalytic acitvity.
The GGDEF domain is named after the highly conserved (in PleD it is GGEEF) that locates to a β-hairpin.
Substrate binding
|
The motif is part of the as identified in the structure of PleD in complex with . The GGDEF domain binds only one GTP substrate molecule. For the reaction to proceed, two GTP loaded GGDEF domains have to align antiparallely.
Complete active site
| Theoretical Model: The protein structure described on this page was determined theoretically, and hence should be interpreted with caution. |
After binding of the (here we show GMP, please update), it is believed that two GGDEF domains associate antiparallely to form a catalytically competent dimer (, ). Then, by inter-molecular nucleophilic in-line attack of the O3' atom onto the α-phosphorous of the other GTP substrate molecule, two phosphodiester bonds are formed under release of two pyrophosphate molecules (2 GTP -> c-di-GMP + 2 PPi).
References
Activated PleD structure 2v0n:
- Wassmann P, Chan C, Paul R, Beck A, Heerklotz H, Jenal U, Schirmer T. Structure of BeF3- -modified response regulator PleD: implications for diguanylate cyclase activation, catalysis, and feedback inhibition. Structure. 2007 Aug;15(8):915-27. PMID:17697997 doi:http://dx.doi.org/10.1016/j.str.2007.06.016
- Paul R, Weiser S, Amiot NC, Chan C, Schirmer T, Giese B, Jenal U. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 2004 Mar 15;18(6):715-27. PMID:15075296 doi:http://dx.doi.org/10.1101/gad.289504
- Paul R, Abel S, Wassmann P, Beck A, Heerklotz H, Jenal U. Activation of the diguanylate cyclase PleD by phosphorylation-mediated dimerization. J Biol Chem. 2007 Oct 5;282(40):29170-7. Epub 2007 Jul 19. PMID:17640875 doi:http://dx.doi.org/10.1074/jbc.M704702200