Huperzine A Complexed with Acetylcholinesterase (Chinese)
From Proteopedia
| Line 15: | Line 15: | ||
==三维结构== | ==三维结构== | ||
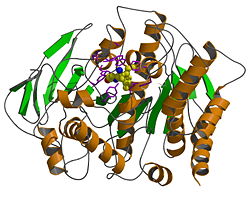
| - | + | 分辨率为2.5埃的石杉碱甲与从加利福尼亚电鳐中提取的乙酰胆碱酯酶复合物的晶体结构于1997年测定(其在蛋白质库中的编码为'''1vot''')。晶体结构表明石杉碱甲以非常出乎意料的取向与乙酰胆碱酯酶结合,与其对乙酰胆碱酯酶高亲合力性质相对应的是石杉碱甲与关键蛋白质残基形成了较强的相互作用。乙酰胆碱酯酶的催化活性位点位于其<scene name='1vot/Active_site/1'>狭长口袋</scene>的底部,<scene name='1vot/Active_site/2'>十四个芳香性残基</scene>坐落在整个口袋的内壁。乙酰胆碱酯酶的天然底物<scene name='1vot/Active_site/3'>乙酰胆碱</scene>与<scene name='1vot/Active_site/5'>催化三联体</scene>(Ser200, His440, and Glu327)之一的残基<scene name='1vot/Active_site/4'>Ser200</scene>直接键连。此外,残基<scene name='1vot/Active_site/6'>Trp84和Phe330</scene>对配体识别结合也非常重要。与乙酰胆碱相似,<font color='blueviolet'><b>石杉碱甲</b></font>也结合于乙酰胆碱酯酶的催化活性位点,其取向基本上与<font color='gray'><b>乙酰胆碱</b></font>的取向<scene name='1vot/Active_site/8'>垂直</scene>。 | |
| + | |||
| + | The principal interactions of <scene name='1vot/1vot_ache_interactions/1'>HupA with TcAChE</scene> are including: a direct <scene name='1vot/1vot_199_130_117/1'>hydrogen bond (HB) with Tyr130 and HBs with Glu199 and Gly117 </scene>through a water molecule as a linker at the bottom of the gorge; cation-pi interactions between the amino group of <scene name='1vot/1vot_84_330/1'>HupA and Trp84 and Phe330</scene> with the distance between the nitrogen and the centroid of the aromatic rings of 4.8 and 4.7 Å, respectively; at the top of the gorge, HBs through two water molecules as linkers formed between the amino group of <scene name='1vot/1vot_70_72_81_85_121/2'>HupA and Tyr70, Asp72, Ser81, Asn85 and Tyr121</scene>. An unusually short (~3.0 Å) C-H→O HB has been seen between the ethylidene methyl group of <scene name='1vot/1vot_440/1'>HupA and the main chain oxygen of His440</scene>. | ||
{{Clear}} | {{Clear}} | ||
Revision as of 11:38, 5 August 2009
石杉碱甲与乙酰胆碱酯酶复合物的三维结构
Contents |
背景介绍
中国科研工作者在20世纪80年代从中药[1]千层塔中分离得到天然产物被证实是乙酰胆碱酯酶的可逆抑制剂,对乙酰胆碱酯酶具有特定、高效的抑制活性。早在1000多年前,中国人已将千层塔用于擦伤、疲惫、肿胀、精神分裂症以及重症肌无力等疾病的治疗。从1996年开始,药品名为双益平[2]的石杉碱甲已在中国广泛用于早老年痴呆症的治疗。与美国食品药品管理局(FDA)批准的目前用于老年痴呆症治疗的多奈哌齐(Donepezil,商品名Aricept)、利伐司替明(Rivastigmine,商品名Exelon)和加兰他敏(Galanthamine,商品名Reminyl)三个药相比,石杉碱甲具有能更好渗透血脑屏障、生物口服利用度更高和对乙酰胆碱酯酶抑制时效更长的特点。
的结构同其他乙酰胆碱酯酶抑制剂和底物具有一定的相似性.整个分子结构比较刚性,包括芳香坏和在生理pH下可能质子化的氨基。在石杉碱甲和乙酰胆碱酯酶复合物的三维结构测定之前,有很多关于石杉碱甲与乙酰胆碱酯酶的作用模式以及其药效团如何与蛋白质残基相互作用等的猜测。因此,该复合物晶体结构的解析可以准确的提供了这些疑问的答案,同时为进一步基于复合物结构而设计出更加有效的石杉碱甲类似物(或衍生物)等乙酰胆碱酯酶抑制剂提供帮助。
|
三维结构
分辨率为2.5埃的石杉碱甲与从加利福尼亚电鳐中提取的乙酰胆碱酯酶复合物的晶体结构于1997年测定(其在蛋白质库中的编码为1vot)。晶体结构表明石杉碱甲以非常出乎意料的取向与乙酰胆碱酯酶结合,与其对乙酰胆碱酯酶高亲合力性质相对应的是石杉碱甲与关键蛋白质残基形成了较强的相互作用。乙酰胆碱酯酶的催化活性位点位于其的底部,坐落在整个口袋的内壁。乙酰胆碱酯酶的天然底物与(Ser200, His440, and Glu327)之一的残基直接键连。此外,残基对配体识别结合也非常重要。与乙酰胆碱相似,石杉碱甲也结合于乙酰胆碱酯酶的催化活性位点,其取向基本上与乙酰胆碱的取向。
The principal interactions of are including: a direct through a water molecule as a linker at the bottom of the gorge; cation-pi interactions between the amino group of with the distance between the nitrogen and the centroid of the aromatic rings of 4.8 and 4.7 Å, respectively; at the top of the gorge, HBs through two water molecules as linkers formed between the amino group of . An unusually short (~3.0 Å) C-H→O HB has been seen between the ethylidene methyl group of .
关于此结构
1vot is a Single protein structure of sequence from Torpedo californica. Full crystallographic information is available from OCA.
参考文献
Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A., Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL, Nat. Struct. Biol. 1997 Jan;4(1):57-63. PMID:8989325
Huperzine A from Huperzia species-An ethnopharmacolgical review., Ma X, Tan C, Zhu D, Gang D, Xiao P, J. Ethnopharmacol. 2007 Aug;113(1):15-34. PMID:17644292
Page seeded by OCA on Mon Mar 31 00:26:42 2008
Proteopedia Page Contributors and Editors (what is this?)
Yechun Xu, Jaime Prilusky, Alexander Berchansky, Joel L. Sussman, Eric Martz