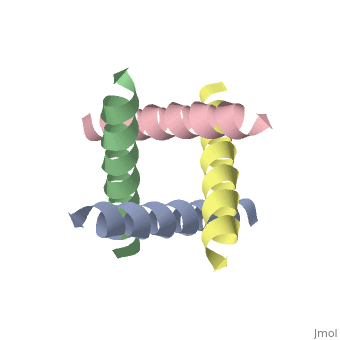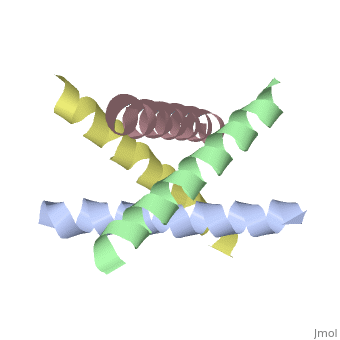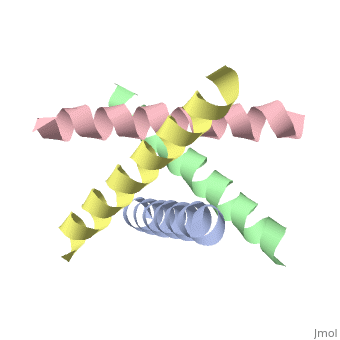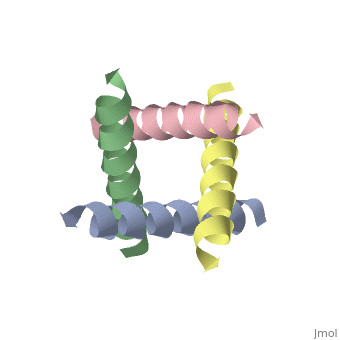User:Amy Kerzmann/Sandbox 4
From Proteopedia
(Difference between revisions)
(New page: == M2 Proton Channel from ''Influenza'' A Virus == <applet load='1nyj' size='300' frame='true' align='right' caption='The closed state structure of M2 protein H+ channel by solid state NMR...) |
(Replacing page with '== M2 Proton Channel from ''Influenza'' A Virus == <applet load='1nyj' size='300' frame='true' align='right' caption='The closed state structure of M2 protein H+ channel by sol...') |
||
| Line 1: | Line 1: | ||
== M2 Proton Channel from ''Influenza'' A Virus == | == M2 Proton Channel from ''Influenza'' A Virus == | ||
| - | <applet load='1nyj' size='300' frame='true' align='right' caption='The closed state structure of M2 protein H+ channel by solid state NMR spectroscopy <ref>PMID:12403618</ref>. | + | <applet load='1nyj' size='300' frame='true' align='right' caption='The closed state structure of M2 protein H+ channel by solid state NMR spectroscopy <ref>PMID:12403618</ref>. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 23:24, 29 September 2009
M2 Proton Channel from Influenza A Virus
| |||||||||||