User:Laura Fountain/Chloride Ion Channel
From Proteopedia
| Line 1: | Line 1: | ||
== CLIC1: A Chloride Ion Channel == | == CLIC1: A Chloride Ion Channel == | ||
| - | CLIC1 (NCC27) is a member of the highly conserved class of chloride ion channels that exist in both soluble and integral membrane forms. The CLIC family consists of seven distinct members: CLIC1, CLIC2, CLIC3, CLIC4, CLIC5, p64, and parchorin. The family is defined by a COOH-terminal core segment of ~230 amino acids that is highly conserved among all family members. CLIC1 has only a few amino acids upstream of this conserved core. CLIC1 is the most commonly studied member of the CLIC family because it is expressed to some extent in most tissues and cell types that have been studied and is particularly highly expressed in muscle.<ref name="Tulk">PMID:11940526</ref>CLIC1 has also been found in various intracellular membranes such as the mitochondrial, nuclear, and endoplasmic reticular membranes.<ref>PMID: | + | CLIC1 (NCC27) is a member of the highly conserved class of chloride ion channels that exist in both soluble and integral membrane forms. The CLIC family consists of seven distinct members: CLIC1, CLIC2, CLIC3, CLIC4, CLIC5, p64, and parchorin. The family is defined by a COOH-terminal core segment of ~230 amino acids that is highly conserved among all family members. CLIC1 has only a few amino acids upstream of this conserved core. CLIC1 is the most commonly studied member of the CLIC family because it is expressed to some extent in most tissues and cell types that have been studied and is particularly highly expressed in muscle.<ref name="Tulk">PMID:11940526</ref>CLIC1 has also been found in various intracellular membranes such as the mitochondrial, nuclear, and endoplasmic reticular membranes.<ref name="Transition">PMID:12202911</ref> |
Because of their wide array of locations within the cell there is still a lot of research being done to discover their various functions within the cell. Some of the possibilities currently listed are: cell signaling, cell division, apoptosis, and, of course, ion flow regulation. | Because of their wide array of locations within the cell there is still a lot of research being done to discover their various functions within the cell. Some of the possibilities currently listed are: cell signaling, cell division, apoptosis, and, of course, ion flow regulation. | ||
| Line 10: | Line 10: | ||
<applet load='1k0o' size='300' frame='true' align='right' caption='Soluble form of CLIC1' /> | <applet load='1k0o' size='300' frame='true' align='right' caption='Soluble form of CLIC1' /> | ||
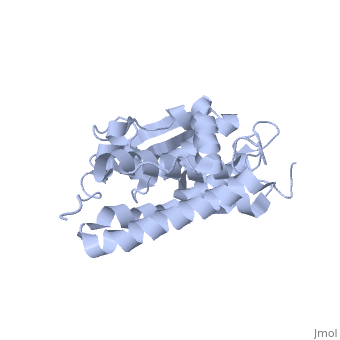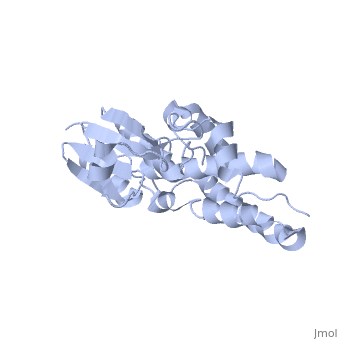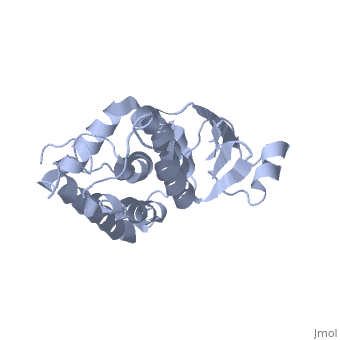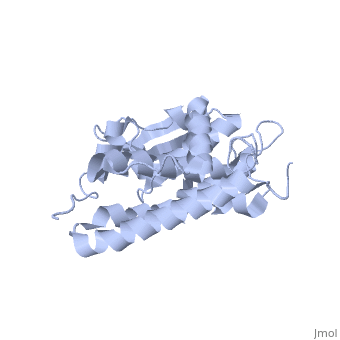
| - | Purified CLIC1 can integrate into synthetic lipid bilayers forming a chloride channel with similar properties to those observed in vivo. The structure of the soluble form of CLIC1 has been determined at 1.4-A resolution, and is shown to the right. It's a homodimeric structure with one pore per subunit, creating a "double barreled" channel. At its binding site in the pore, chloride interacts with the ends of four helices that come from both sides of the membrane. A <scene name='User:Laura_Fountain/Sandbox_1/Glutamate_residue/1'>glutamate residue</scene> that protrudes into the pore is proposed to participate in gating.<ref>PMID: | + | Purified CLIC1 can integrate into synthetic lipid bilayers forming a chloride channel with similar properties to those observed in vivo. The structure of the soluble form of CLIC1 has been determined at 1.4-A resolution, and is shown to the right. It's a homodimeric structure with one pore per subunit, creating a "double barreled" channel. At its binding site in the pore, chloride interacts with the ends of four helices that come from both sides of the membrane. A <scene name='User:Laura_Fountain/Sandbox_1/Glutamate_residue/1'>glutamate residue</scene> that protrudes into the pore is proposed to participate in gating.<ref name="CLC">PMID:12163078</ref> Integration of CLIC1 into the membrane is likely to require a major structural rearrangement, probably of the N-domain (<scene name='User:Laura_Fountain/Sandbox_1/N-domain/3'>residues 1-90</scene>), with the putative transmembrane helix arising from residues in the vicinity of the redox-active site.<ref name="Crystal">PMID:11551966</ref> |
| - | While this exact mechanism isn't known, it has been shown that functionality of the channel doesn't change whether it goes through 'normal' membrane integration via vesicles, or whether it's inserted into the intracellular space and allowed to integrate itself.<ref name="Tulk">PMID: | + | While this exact mechanism isn't known, it has been shown that functionality of the channel doesn't change whether it goes through 'normal' membrane integration via vesicles, or whether it's inserted into the intracellular space and allowed to integrate itself.<ref name="Tulk">PMID:11940526</ref> Littler et. al. propose that upon oxidation CLIC1 undergoes a reversible transition from a monomeric to a non-covalent dimeric state due to the formation of an intramolecular disulfide bond (<scene name='User:Laura_Fountain/Sandbox_1/Cys_visualization/1'>Cys-24-Cys-59</scene>). They have determined the crystal structure of this oxidized state and show that a major structural transition has occurred, exposing a large hydrophobic surface, which forms the dimer interface. The oxidized CLIC1 dimer maintains its ability to form chloride ion channels in artificial bilayers and vesicles, whereas a reducing environment prevents the formation of ion channels by CLIC1. Their mutational studies show that both Cys-24 and Cys-59 are required for channel activity.<ref name="Intracellular">PMID:14613939</ref> |
| - | 1K0O is a 2 chains structure of sequences from Homo sapiens. The protein is monomeric and structurally homologous to the glutathione S-transferase superfamily, and it has a redox-active site resembling glutaredoxin. The structure of the complex of CLIC1 with glutathione shows that glutathione occupies the redox-active site, which is adjacent to an open, elongated slot lined by basic residues. This structure indicates that CLIC1 is likely to be controlled by redox-dependent processes.<ref>PMID: | + | 1K0O is a 2 chains structure of sequences from Homo sapiens. The protein is monomeric and structurally homologous to the glutathione S-transferase superfamily, and it has a redox-active site resembling glutaredoxin. The structure of the complex of CLIC1 with glutathione shows that glutathione occupies the redox-active site, which is adjacent to an open, elongated slot lined by basic residues. This structure indicates that CLIC1 is likely to be controlled by redox-dependent processes.<ref name="Crystal">PMID:11551966</ref> |
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 03:47, 5 October 2009
CLIC1: A Chloride Ion Channel
CLIC1 (NCC27) is a member of the highly conserved class of chloride ion channels that exist in both soluble and integral membrane forms. The CLIC family consists of seven distinct members: CLIC1, CLIC2, CLIC3, CLIC4, CLIC5, p64, and parchorin. The family is defined by a COOH-terminal core segment of ~230 amino acids that is highly conserved among all family members. CLIC1 has only a few amino acids upstream of this conserved core. CLIC1 is the most commonly studied member of the CLIC family because it is expressed to some extent in most tissues and cell types that have been studied and is particularly highly expressed in muscle.[1]CLIC1 has also been found in various intracellular membranes such as the mitochondrial, nuclear, and endoplasmic reticular membranes.[2]
Because of their wide array of locations within the cell there is still a lot of research being done to discover their various functions within the cell. Some of the possibilities currently listed are: cell signaling, cell division, apoptosis, and, of course, ion flow regulation.
About this Structure
|
Purified CLIC1 can integrate into synthetic lipid bilayers forming a chloride channel with similar properties to those observed in vivo. The structure of the soluble form of CLIC1 has been determined at 1.4-A resolution, and is shown to the right. It's a homodimeric structure with one pore per subunit, creating a "double barreled" channel. At its binding site in the pore, chloride interacts with the ends of four helices that come from both sides of the membrane. A that protrudes into the pore is proposed to participate in gating.[3] Integration of CLIC1 into the membrane is likely to require a major structural rearrangement, probably of the N-domain (), with the putative transmembrane helix arising from residues in the vicinity of the redox-active site.[4]
While this exact mechanism isn't known, it has been shown that functionality of the channel doesn't change whether it goes through 'normal' membrane integration via vesicles, or whether it's inserted into the intracellular space and allowed to integrate itself.[1] Littler et. al. propose that upon oxidation CLIC1 undergoes a reversible transition from a monomeric to a non-covalent dimeric state due to the formation of an intramolecular disulfide bond (). They have determined the crystal structure of this oxidized state and show that a major structural transition has occurred, exposing a large hydrophobic surface, which forms the dimer interface. The oxidized CLIC1 dimer maintains its ability to form chloride ion channels in artificial bilayers and vesicles, whereas a reducing environment prevents the formation of ion channels by CLIC1. Their mutational studies show that both Cys-24 and Cys-59 are required for channel activity.[5]
1K0O is a 2 chains structure of sequences from Homo sapiens. The protein is monomeric and structurally homologous to the glutathione S-transferase superfamily, and it has a redox-active site resembling glutaredoxin. The structure of the complex of CLIC1 with glutathione shows that glutathione occupies the redox-active site, which is adjacent to an open, elongated slot lined by basic residues. This structure indicates that CLIC1 is likely to be controlled by redox-dependent processes.[4]
References
- ↑ 1.0 1.1 Tulk BM, Kapadia S, Edwards JC. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am J Physiol Cell Physiol. 2002 May;282(5):C1103-12. PMID:11940526 doi:10.1152/ajpcell.00402.2001
- ↑ Cromer BA, Morton CJ, Board PG, Parker MW. From glutathione transferase to pore in a CLIC. Eur Biophys J. 2002 Sep;31(5):356-64. Epub 2002 May 23. PMID:12202911 doi:10.1007/s00249-002-0219-1
- ↑ Estevez R, Jentsch TJ. CLC chloride channels: correlating structure with function. Curr Opin Struct Biol. 2002 Aug;12(4):531-9. PMID:12163078
- ↑ 4.0 4.1 Harrop SJ, DeMaere MZ, Fairlie WD, Reztsova T, Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Jankova L, Warton K, Bauskin AR, Wu WM, Pankhurst S, Campbell TJ, Breit SN, Curmi PM. Crystal structure of a soluble form of the intracellular chloride ion channel CLIC1 (NCC27) at 1.4-A resolution. J Biol Chem. 2001 Nov 30;276(48):44993-5000. Epub 2001 Sep 10. PMID:11551966 doi:10.1074/jbc.M107804200
- ↑ Littler DR, Harrop SJ, Fairlie WD, Brown LJ, Pankhurst GJ, Pankhurst S, DeMaere MZ, Campbell TJ, Bauskin AR, Tonini R, Mazzanti M, Breit SN, Curmi PM. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J Biol Chem. 2004 Mar 5;279(10):9298-305. Epub 2003 Nov 12. PMID:14613939 doi:http://dx.doi.org/10.1074/jbc.M308444200