Sandbox 1b41
From Proteopedia
| Line 1: | Line 1: | ||
==Human Acetylcholinesterase (1b41)== | ==Human Acetylcholinesterase (1b41)== | ||
{{STRUCTURE_1b41 | PDB=1b41 | SCENE= }} | {{STRUCTURE_1b41 | PDB=1b41 | SCENE= }} | ||
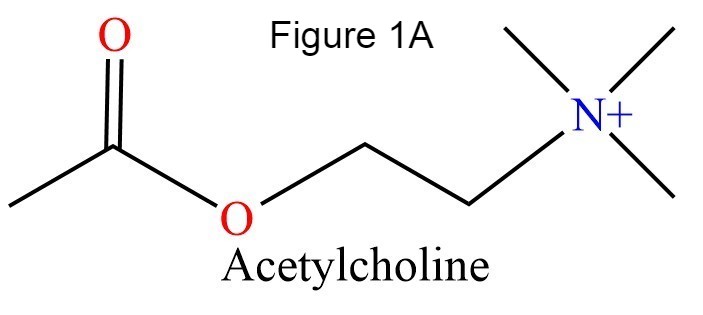
| - | The human acetylcholinesterase (AChE) is an enzyme which hydrolyses the neurotransmitter Acethylcholin (ACh) in the neuromuscular junctions and in other cholinergic synapses to terminate the neuronal signal. | + | The human acetylcholinesterase (AChE) is an enzyme which hydrolyses the neurotransmitter Acethylcholin (ACh) in the neuromuscular junctions and in other cholinergic synapses to terminate the neuronal signal. |
| - | In the physiological conditions, AChE exists as tetramers associated with either collagen-like Q subunit (ColQ) or proline-rich membrane-anchoring protein (PRiMA). There is also a monomeric form which is soluble in the blood. | + | It has an ellipsoidal shape with dimensions ~ 4,5nm x 6nm x 6,5nm. It consists of 12-stranded, central mixed β-sheet surrounded by 14 ά helices. |
| + | |||
| + | In the physiological conditions, AChE exists as tetramers associated with either collagen-like Q subunit (ColQ) or proline-rich membrane-anchoring protein (PRiMA). The AChE is linked with these anchoring molecules by a "the tryptophan amphiphilic tetramerization" domain (WAT). There is also a monomeric form which is soluble in the blood. | ||
[[Image:Acetylcholine.jpg]] | [[Image:Acetylcholine.jpg]] | ||
| + | |||
| + | ==The Active site gorge of AChE== | ||
| + | |||
| + | The active site of AChE involved two sites: the peripheral site and the catalytic site (Figure ). | ||
| + | |||
| + | The peripheral site is a transitional binding site of the substrate. It provides three conserved aromatic residues (Tyr, Trp, Tyr) that guide the ligands (ACh or other agonists) by setting an array of low-affinity binding sites. | ||
Revision as of 11:26, 5 November 2009
Human Acetylcholinesterase (1b41)
| |||||||||
| 1b41, resolution 2.76Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Gene: | ACHE (Homo sapiens) | ||||||||
| Activity: | Acetylcholinesterase, with EC number 3.1.1.7 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
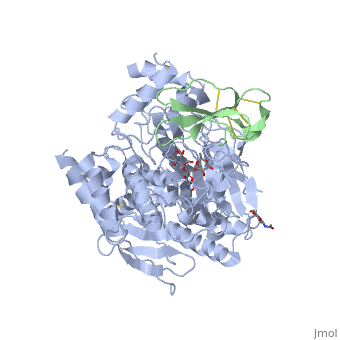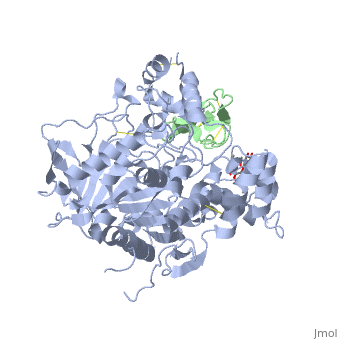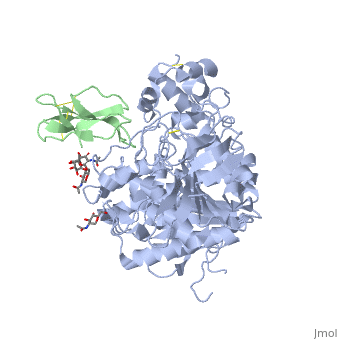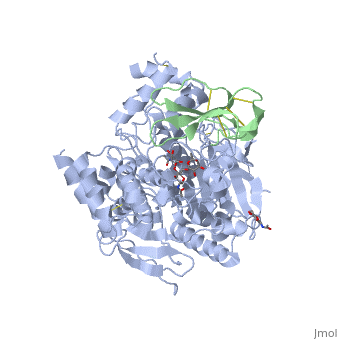
The human acetylcholinesterase (AChE) is an enzyme which hydrolyses the neurotransmitter Acethylcholin (ACh) in the neuromuscular junctions and in other cholinergic synapses to terminate the neuronal signal. It has an ellipsoidal shape with dimensions ~ 4,5nm x 6nm x 6,5nm. It consists of 12-stranded, central mixed β-sheet surrounded by 14 ά helices.
In the physiological conditions, AChE exists as tetramers associated with either collagen-like Q subunit (ColQ) or proline-rich membrane-anchoring protein (PRiMA). The AChE is linked with these anchoring molecules by a "the tryptophan amphiphilic tetramerization" domain (WAT). There is also a monomeric form which is soluble in the blood.
The Active site gorge of AChE
The active site of AChE involved two sites: the peripheral site and the catalytic site (Figure ).
The peripheral site is a transitional binding site of the substrate. It provides three conserved aromatic residues (Tyr, Trp, Tyr) that guide the ligands (ACh or other agonists) by setting an array of low-affinity binding sites.