Beta-glucosidase
From Proteopedia
| Line 20: | Line 20: | ||
Here the protein is presented in complex with an inhibitor called <scene name='Sandbox_155/Ligand/1'>calystegine</scene>. We can see that the two glutamate residues we talked about before are really closed to the ligand. Indeed there are interactions between those residues and calystegine | Here the protein is presented in complex with an inhibitor called <scene name='Sandbox_155/Ligand/1'>calystegine</scene>. We can see that the two glutamate residues we talked about before are really closed to the ligand. Indeed there are interactions between those residues and calystegine | ||
| + | |||
== Other use of β-glucosidases == | == Other use of β-glucosidases == | ||
Revision as of 10:19, 8 November 2009
Contents |
Introduction
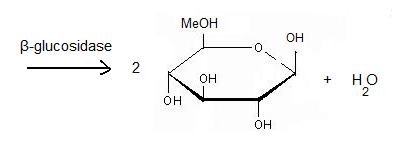
2VRJ is a β-glucosidase which EC number is 3.2.1.21. It comes from Thermotoga maritima which is a rod-shaped bacterium belonging to the order of Thermotogates. This bacterium was originally isolated from geothermal heated marine sediments. A β-glucosidase is an enzyme which catalyses the hydrolysis of terminal non-reducing residues in β-glucosides. It acts on the β(1-4) bond linking two glucose residues or glucose-substituted molecules. The action of the enzyme on such glucosides results in the release of one glucose unit.
In terms of structure 2VRJ is a homodimer. It means that it is made of two chains (A and B) which are identical. Each chain is made of 438 residues and constitutes a subunit of the protein. Each subunit contains a catalytic site.
2VRJ: Structure/function
The enzymatic hydrolysis of a glycosidic bond requires two critical residues : a proton donor and a proton acceptor which can also be called a nucleophile/base. Aspartate and glutamate have been found to perform catalysis. β-glucosidases are exocellulases. It means that they act only on the end of the molecule they have to hydrolyse. It implies that the catalytic site has to be near the surface of the protein so that the residues which compose the catalytic site have to be hydrophilic. Glutamate and asparagin are hydrophilic amino-acids so it corresponds. 2VRJ presents two catalytic sites composed of 3 residues : 2 residues of glutamate () and one residue of asparagin ().
Here the protein is presented in complex with an inhibitor called . We can see that the two glutamate residues we talked about before are really closed to the ligand. Indeed there are interactions between those residues and calystegine
Other use of β-glucosidases
β-glucosidase is now used for the synthesis of biofuel. Wood is an abundant and renewable energy which an be changed into bioethanol thanks to enzyme hydrolysis. This synthesis needs 5 steps. First it is pre-hydrolysis. The structure is divided into lignin and (hemi)cellulose. Cellulase, the enzyme can better access the structure to act on it. The second step: hydrolysis is the most important. Cellulase is a complex of 3 enzymes which act together to hydrolyse cellulose: Endoglucanase breaks the chain in the middle of the molecular structure of cellulose. Exoglucanase binds an available end of the chain and isolates it. Then units of cellobiose are cut( 2 units od glucose which are together ). To finish, β-glucosidase divides cellobiose into 2 glucoses. When they ferment, they become ethanol. The final product is obtained thanks to fermentation, distillation and deshydratation.
References
http://www.cazy.org/fam/ghf_INV_RET.html#3 http://www.ebi.ac.uk/pdbe-srv/view/entry/2vrj/viewer http://www3.interscience.wiley.com/cgi-bin/fulltext/121428480/HTMLSTART
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Muriel Breteau, Alexander Berchansky, Joel L. Sussman, David Canner