Sandbox122
From Proteopedia
| Line 10: | Line 10: | ||
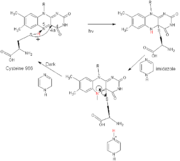
The <scene name='Sandbox122/Fmn_ligand/1'>FMN</scene> (Flavin MonoNucleotide) is the ligand which is responsible for the light absorption. A single molecule of FMN is bound non convalently in the interior of LOV2 domain. FMN is stabilized thanks to hydrogen bonds, Van der Waals and electrostatic interactions. For example, atoms <scene name='Sandbox122/Arg_983/1'>R983</scene> and <scene name='Sandbox122/Arg_963/1'>R967</scene>(alpha C helix) create ionic bond with phosphate group of FMN. <scene name='Sandbox122/Q970/1'>Q970</scene>, <scene name='Sandbox122/N965/1'>N965</scene> , <scene name='Sandbox122/N998/1'>N988</scene>, <scene name='Sandbox122/N1008/1'>N1008</scene> (alpha A helix and beta-strand C, D and E) aminoacid make some electrostatic interaction which stabilize FMN. Some results indicate that the majority of the FMN in the LOV2 domain exist in the protonated form. Researcher propose a reaction mechanism that involves excited-state proton transfer, on the nanosecond time scale , from the sulfhydryl group of the conserved cysteine to the N5 atom of FMN. | The <scene name='Sandbox122/Fmn_ligand/1'>FMN</scene> (Flavin MonoNucleotide) is the ligand which is responsible for the light absorption. A single molecule of FMN is bound non convalently in the interior of LOV2 domain. FMN is stabilized thanks to hydrogen bonds, Van der Waals and electrostatic interactions. For example, atoms <scene name='Sandbox122/Arg_983/1'>R983</scene> and <scene name='Sandbox122/Arg_963/1'>R967</scene>(alpha C helix) create ionic bond with phosphate group of FMN. <scene name='Sandbox122/Q970/1'>Q970</scene>, <scene name='Sandbox122/N965/1'>N965</scene> , <scene name='Sandbox122/N998/1'>N988</scene>, <scene name='Sandbox122/N1008/1'>N1008</scene> (alpha A helix and beta-strand C, D and E) aminoacid make some electrostatic interaction which stabilize FMN. Some results indicate that the majority of the FMN in the LOV2 domain exist in the protonated form. Researcher propose a reaction mechanism that involves excited-state proton transfer, on the nanosecond time scale , from the sulfhydryl group of the conserved cysteine to the N5 atom of FMN. | ||
Blue light arrives on the only LOV2 <scene name='Sandbox122/Cystein_residue/1'>cystein residue</scene>(situate 4.2A from atom C(4a)) and induces formation of covalent cysteinyl-C(4a)adduct. (To explain that, refer to the scheme). Somes studies showed that temperature have an influence on that adduct. In fact, at low temperature, the microenvironment determine the reactivity of the S-H group of Cys966. A base-catalyzed mechanism for dark state recovery. Imidazole snatch proton from N5 atom of FMN and give it to the cysteine again (explain on the scheme). | Blue light arrives on the only LOV2 <scene name='Sandbox122/Cystein_residue/1'>cystein residue</scene>(situate 4.2A from atom C(4a)) and induces formation of covalent cysteinyl-C(4a)adduct. (To explain that, refer to the scheme). Somes studies showed that temperature have an influence on that adduct. In fact, at low temperature, the microenvironment determine the reactivity of the S-H group of Cys966. A base-catalyzed mechanism for dark state recovery. Imidazole snatch proton from N5 atom of FMN and give it to the cysteine again (explain on the scheme). | ||
| + | ==LOV2 signal transmission== | ||
| + | studies have implicated a role for the central β-sheet scaffold in propagating the signal generated within the FMN-binding pocket to protein changes at the LOV2 surface, which are necessary for activation of the C-terminal kinase domain.21,22 Specifically, the side chain of a conserved glutamine residue within LOV2 (Gln575 in Arabidopsis phot1) which forms hydrogen bonds with the FMN chromophore flips by 180° upon cysteinyl adduct formation11,23,24 causing protein changes in the central β-sheet scaffold that forms contacts with the Jα-helix. | ||
This complex may interact directly with the kinase and regulate his activity. | This complex may interact directly with the kinase and regulate his activity. | ||
| + | ==Kinase regulation by LOV2== | ||
| + | The current view of phototropin receptor activation is that LOV2 functions as a repressor of the C-terminal kinase domain in the dark and that this mode of repression is alleviated upon photoexcitation, resulting in receptor autophosphorylation.The predominant role of LOV2 in regulating phototropin activity appears to arise from its position within the phototropin molecule. Photoexcitation of an extended LOV2 fragment leads to displacement of an α-helix from the surface of the LOV2-core. | ||
Kinase will permit the autophosphorylation of phototropin. The rate of phosphorylatide phototropin acts to differential lateral gradients of auxin which is responsible of phototropism phenomenon. | Kinase will permit the autophosphorylation of phototropin. The rate of phosphorylatide phototropin acts to differential lateral gradients of auxin which is responsible of phototropism phenomenon. | ||
| + | For more information about the continuation of this mechanism refer to this PDB protein: 2z6c,2z6d, | ||
[[Image:Noname05.gif | thumb]] | [[Image:Noname05.gif | thumb]] | ||
[[Image:1G28.pdb_b.jpg | thumb]] | [[Image:1G28.pdb_b.jpg | thumb]] | ||
Revision as of 17:43, 10 November 2009
|
Phototropin is a blue light receptor involved in the phototropism (it is a phenomenon which is growth directed by light).This is a transmembrane protein present on the top of coleoptile. This protein is a dimer (phot1 and phot2) and each subunit contains three domains : LOV1, LOV2 (light,oxygen or voltage) domains and a kinase domain.
LOV2 domain was, here, chosen for study.
Contents |
Structure of LOV2
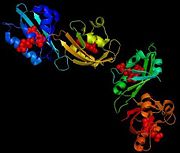
The structure of a flaving domain, LOV2, from the chimeric phototropin photoreceptor PHY3, has been determined thanks to X-Ray diffraction. This domain is composed by four chains which are identical A, B, C and D. It's a L-polypeptide which weight 50552,99 Da. Each chains has 104 aminoacids and possess four (28 residues which represents 26%) and six B (37 residues which represent 35%).
Interaction between FMN et LOV2 domain
The (Flavin MonoNucleotide) is the ligand which is responsible for the light absorption. A single molecule of FMN is bound non convalently in the interior of LOV2 domain. FMN is stabilized thanks to hydrogen bonds, Van der Waals and electrostatic interactions. For example, atoms and (alpha C helix) create ionic bond with phosphate group of FMN. , , , (alpha A helix and beta-strand C, D and E) aminoacid make some electrostatic interaction which stabilize FMN. Some results indicate that the majority of the FMN in the LOV2 domain exist in the protonated form. Researcher propose a reaction mechanism that involves excited-state proton transfer, on the nanosecond time scale , from the sulfhydryl group of the conserved cysteine to the N5 atom of FMN. Blue light arrives on the only LOV2 (situate 4.2A from atom C(4a)) and induces formation of covalent cysteinyl-C(4a)adduct. (To explain that, refer to the scheme). Somes studies showed that temperature have an influence on that adduct. In fact, at low temperature, the microenvironment determine the reactivity of the S-H group of Cys966. A base-catalyzed mechanism for dark state recovery. Imidazole snatch proton from N5 atom of FMN and give it to the cysteine again (explain on the scheme).
LOV2 signal transmission
studies have implicated a role for the central β-sheet scaffold in propagating the signal generated within the FMN-binding pocket to protein changes at the LOV2 surface, which are necessary for activation of the C-terminal kinase domain.21,22 Specifically, the side chain of a conserved glutamine residue within LOV2 (Gln575 in Arabidopsis phot1) which forms hydrogen bonds with the FMN chromophore flips by 180° upon cysteinyl adduct formation11,23,24 causing protein changes in the central β-sheet scaffold that forms contacts with the Jα-helix.
This complex may interact directly with the kinase and regulate his activity.
Kinase regulation by LOV2
The current view of phototropin receptor activation is that LOV2 functions as a repressor of the C-terminal kinase domain in the dark and that this mode of repression is alleviated upon photoexcitation, resulting in receptor autophosphorylation.The predominant role of LOV2 in regulating phototropin activity appears to arise from its position within the phototropin molecule. Photoexcitation of an extended LOV2 fragment leads to displacement of an α-helix from the surface of the LOV2-core. Kinase will permit the autophosphorylation of phototropin. The rate of phosphorylatide phototropin acts to differential lateral gradients of auxin which is responsible of phototropism phenomenon. For more information about the continuation of this mechanism refer to this PDB protein: 2z6c,2z6d,