User:Claire Roudot/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <applet load='2rnm' size='450' frame='true' align='right' caption='2rnm' | + | <applet load='2rnm' size='450' frame='true' align='right' caption='2rnm' > |
| - | == Amyloid Fibrils of the HET-s(218-289) Prion Form== | ||
| - | -- | + | == Amyloid Fibrils of the HET-s(218-289) Prion Form== |
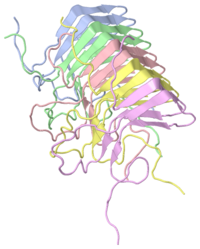
The '''HET-s protein''' from the filamentous fungus Podospora Anserina has a prion forming domain from residues 218 to 289. | The '''HET-s protein''' from the filamentous fungus Podospora Anserina has a prion forming domain from residues 218 to 289. | ||
| Line 9: | Line 8: | ||
Prions are proteins Prp-sc which have a different domain conformation than the natural protein Prp-c. | Prions are proteins Prp-sc which have a different domain conformation than the natural protein Prp-c. | ||
The prion form of HET-s plays a role in heterokaryon incompatibility, a fungal self/nonself recognition phenomenon that prevents different form of parasitism. | The prion form of HET-s plays a role in heterokaryon incompatibility, a fungal self/nonself recognition phenomenon that prevents different form of parasitism. | ||
| + | |||
| + | |||
| Line 14: | Line 15: | ||
| - | Structure | + | |
| + | |||
| + | |||
| + | |||
| + | == Structure== | ||
| + | |||
The global organization of the HET-s (218-289) fibril is a left handed β solenoid with two windings per molecule. The studies of this part of molecule have been done with NMR. | The global organization of the HET-s (218-289) fibril is a left handed β solenoid with two windings per molecule. The studies of this part of molecule have been done with NMR. | ||
HET-s (218-289) is formed by four β strands, each strand is formed two parallel β sheet with short HN-Hα contacts (3,0A). Three strands constitute the core of the fibril. The last strand is outside the core and is formed by β2b and β4b. The segments β1a and β1b (β3a and β3b) have an approximately rectangular kink between them thanks a connection by a two-residue β arc, changing the inside-outside pattern of side chains. The connection between β1b and β2a (and similarly, between β3b and β4a) is linked by a three-residue β arc, allowing for the orientation change of the polypeptide backbone by ~ 150°. The β-sheet pattern is disrupted between β2a and β2b, leading to ~90° arcs. β1-β2 and β3-β4 are pseudo repeats and linked by parallel intramolecular and intermolecular H bonds, as follows: β1a-β3a, β1b-β3b, β2a-β4a, β2b-β4b. | HET-s (218-289) is formed by four β strands, each strand is formed two parallel β sheet with short HN-Hα contacts (3,0A). Three strands constitute the core of the fibril. The last strand is outside the core and is formed by β2b and β4b. The segments β1a and β1b (β3a and β3b) have an approximately rectangular kink between them thanks a connection by a two-residue β arc, changing the inside-outside pattern of side chains. The connection between β1b and β2a (and similarly, between β3b and β4a) is linked by a three-residue β arc, allowing for the orientation change of the polypeptide backbone by ~ 150°. The β-sheet pattern is disrupted between β2a and β2b, leading to ~90° arcs. β1-β2 and β3-β4 are pseudo repeats and linked by parallel intramolecular and intermolecular H bonds, as follows: β1a-β3a, β1b-β3b, β2a-β4a, β2b-β4b. | ||
| Line 21: | Line 27: | ||
The triangular hydrophobic core formed by three stands has some resemblance with β-solenoid structures of soluble proteins like filamentous hemagglutinin protein. In this case, the periodicity does not exist but he triangular core is quite similar. A β-solenoid fold has also been proposed for the prion state of human prion protein Prp (…..). | The triangular hydrophobic core formed by three stands has some resemblance with β-solenoid structures of soluble proteins like filamentous hemagglutinin protein. In this case, the periodicity does not exist but he triangular core is quite similar. A β-solenoid fold has also been proposed for the prion state of human prion protein Prp (…..). | ||
| + | |||
| + | == Consequences of the structure on the amyloid fibril == | ||
This well-organized structure of the HET-s prion fibrils is originally from the high order is these fibrils as well as the absence of polymorphism caused by different underlying molecular at physiological pH conditions. Indeed interaction between charges in the fibril gives a high stability, so polymorphism is excluding. The fibril structure of HET-s (218-289) along an axe amplified the well-defined structure of a functional amyloid. | This well-organized structure of the HET-s prion fibrils is originally from the high order is these fibrils as well as the absence of polymorphism caused by different underlying molecular at physiological pH conditions. Indeed interaction between charges in the fibril gives a high stability, so polymorphism is excluding. The fibril structure of HET-s (218-289) along an axe amplified the well-defined structure of a functional amyloid. | ||
Revision as of 08:40, 12 November 2009
| |||||||||||