User:Stéphanie Kraemer/Sandbox 106
From Proteopedia
| Line 9: | Line 9: | ||
M2 and p53R2 interact with M1 through the C-terminal binding domain. These two subunits share more than 80% sequence identity. But the few differences between the two are not unimportant, as it’s explained below. | M2 and p53R2 interact with M1 through the C-terminal binding domain. These two subunits share more than 80% sequence identity. But the few differences between the two are not unimportant, as it’s explained below. | ||
The first X-ray crystal structure of p53R2 has a resolution of 2,6 Å and permits to describe its structure and also to show the structural differences with the M2 subunit. | The first X-ray crystal structure of p53R2 has a resolution of 2,6 Å and permits to describe its structure and also to show the structural differences with the M2 subunit. | ||
| + | |||
| + | Structure and function | ||
| + | ---- | ||
Revision as of 19:15, 12 November 2009
p53R2
| |||||||
| 3hf1, resolution 2.60Å () | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||
| Gene: | RRM2B, P53R2 (Homo sapiens) | ||||||
| Activity: | Ribonucleoside-diphosphate reductase, with EC number 1.17.4.1 | ||||||
| Related: | 1xsm, 2uw2, 1w69, 1w68 | ||||||
| |||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
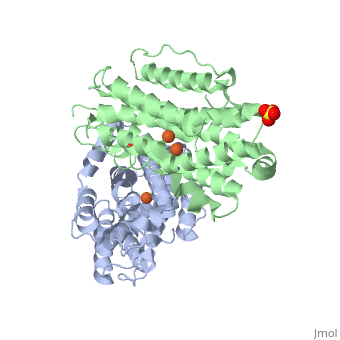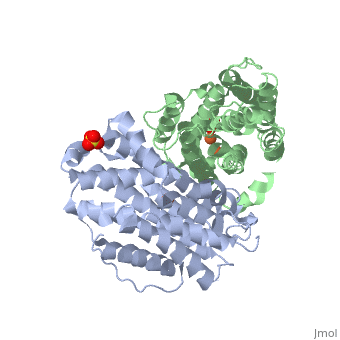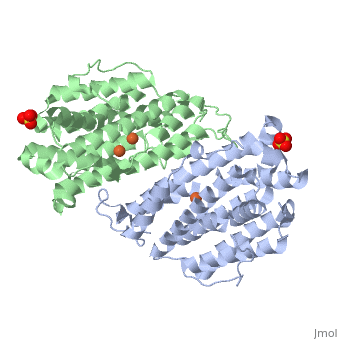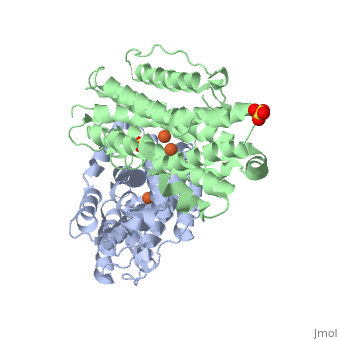
P53R2 is an oxydoreductase composed of 351 residues. It is a small subunit of the ribonucleotide reductase (RNR). RNR catalyses the reduction of the four nucleotides to desoxyribonucleotides. It exists three classes of RNR. Class I RNR is a tetramer composed of the two types of subunits with stoichiometry α2β2 and three subunits have been identified in mammals : - Large (α) subunit M1 that contains the enzyme active site - Small (β) subunit M2 that contains a dinuclear iron site, it’s the regulatory subunit - P53R2, the last identified (in 2000), which is transactivated by p53 in response to DNA damage in cells during the G0-G1 cell cycle phase M2 and p53R2 interact with M1 through the C-terminal binding domain. These two subunits share more than 80% sequence identity. But the few differences between the two are not unimportant, as it’s explained below. The first X-ray crystal structure of p53R2 has a resolution of 2,6 Å and permits to describe its structure and also to show the structural differences with the M2 subunit.
Structure and function