Urate Oxidase
From Proteopedia
| Line 5: | Line 5: | ||
== Introduction == | == Introduction == | ||
| - | Urate oxidase (3bk8) is an enzyme belonging to the purine degradation pathway, whose function is to prevent build-up of uric acid in the blood by catalyzing the oxidation of uric acid by molecular oxygen, producing 5-hydroxyisourate and hydrogen peroxide. The catalytic mechanism of urate oxidase is unique for an oxidase because it does require a cofactor or metal ion. This enzyme is found in many organisms, but not found in higher apes and humans. It has been suggested that this may be an evolutionary advantage, as uric acid is a strong anti-oxidant. This leads to the presence of fewer free radicals and therefore fewer instances of cancer as a result of aging. The lack of urate oxidase does, however, result in elevated levels of uric acid in the plasma, which can be fatal. Urate oxidase was first isolated from the fungus ''Aspergillus flavus'', but is now expressed in ''Saccharomyces cerevisiae'' for research and medical purposes. | + | Urate oxidase (3bk8) is an enzyme belonging to the purine degradation pathway, whose function is to prevent build-up of uric acid in the blood by catalyzing the oxidation of uric acid by molecular oxygen, producing 5-hydroxyisourate and hydrogen peroxide. The catalytic mechanism of urate oxidase is unique for an oxidase because it does not require a cofactor or metal ion. This enzyme is found in many organisms, but not found in higher apes and humans. It has been suggested that this may be an evolutionary advantage, as uric acid is a strong anti-oxidant. This leads to the presence of fewer free radicals and therefore fewer instances of cancer as a result of aging. The lack of urate oxidase does, however, result in elevated levels of uric acid in the plasma, which can be fatal. Urate oxidase was first isolated from the fungus ''Aspergillus flavus'', but is now expressed in ''Saccharomyces cerevisiae'' for research and medical purposes. |
== Structure == | == Structure == | ||
Revision as of 00:47, 16 March 2010
Contents |
Urate Oxidase
|
Introduction
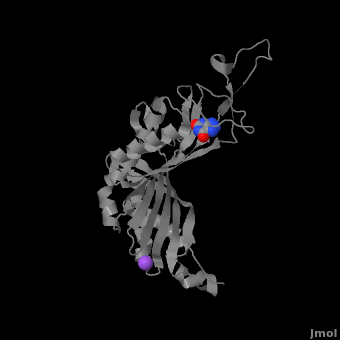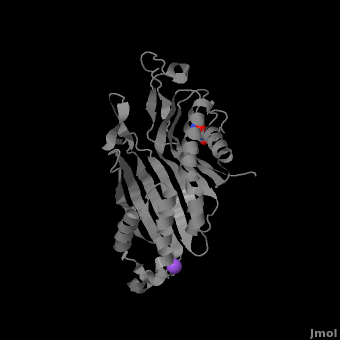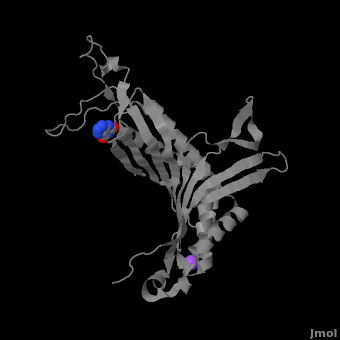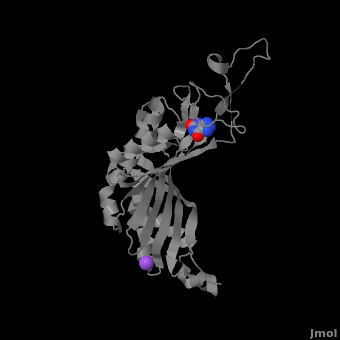
Urate oxidase (3bk8) is an enzyme belonging to the purine degradation pathway, whose function is to prevent build-up of uric acid in the blood by catalyzing the oxidation of uric acid by molecular oxygen, producing 5-hydroxyisourate and hydrogen peroxide. The catalytic mechanism of urate oxidase is unique for an oxidase because it does not require a cofactor or metal ion. This enzyme is found in many organisms, but not found in higher apes and humans. It has been suggested that this may be an evolutionary advantage, as uric acid is a strong anti-oxidant. This leads to the presence of fewer free radicals and therefore fewer instances of cancer as a result of aging. The lack of urate oxidase does, however, result in elevated levels of uric acid in the plasma, which can be fatal. Urate oxidase was first isolated from the fungus Aspergillus flavus, but is now expressed in Saccharomyces cerevisiae for research and medical purposes.
Structure
The complete functional structure of urate oxidase is a 135-kDa barrel-shaped homo-tetramer that has a height of 7 nm, with an inner radius of 0.6 nm and an outer radius of 3 nm. It has four identical active sites that are found at dimeric interfaces formed between the monomers and a central void tunnel of unknown function that is 5 nm long and has a diameter of 1.2 nm. Each monomer, formed by 301 amino acid residues, has two structuarally equivalent domains, which Collac`h et al. termed ``tunneling fold`` domains (this feature allows urate oxidase to be placed in the expanding family of tunnel-shaped proteins that also includes 3i2b and 1a9c). These domains are comprised of a four-strand long antiparallel beta sheet with two helices on the concave side. Together, the two tunneling fold domains of each monomer form an eight-stand long antiparallel beta sheet, where all four helices are found on the concave side of the sheet. The dimer forms an α8β16 barrel, in which the eight helices make up the outer surface of the barrel. The active site
Function
Medical Implications
Hyperuricemia is a condition in which uric acid levels in the blood become abnormally high. This may be caused by various factors including genetic disorders (Lesch-Nyhan syndrome), diet, kidney disease, and chemotherapy. Symptoms of hyperuricemia include joint inflammation (gout), kidney stones, pain and fever. In some patients, chemotherapy induces a condition known as tumor lysis syndrome (essentially chemotherapy-induced hyperuricemia). Tumor lysis syndrome results from the rapid breakdown of cells that occurs after chemotherapy, leading to significantly increased levels of uric acid in the blood. Sanofi-Aventis, a European-based pharmaceutical company, produces a drug known as Fasturtec (rasburicase) that is a recombinant form of urate oxidase that is produced in a genetically modified strain of Saccharomyces cerevisiae. This drug is administered to patients who are experiencing tumor lysis syndrome as a result of chemotherapy.
References
<tr><td>Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell.</td></tr> Sonja Senekovic
Proteopedia Page Contributors and Editors (what is this?)
Sonja Senekovic, Michal Harel, Alexander Berchansky, David Canner, Andrea Gorrell, Joel L. Sussman