User:Tilman Schirmer/Sandbox 99
From Proteopedia
| Line 6: | Line 6: | ||
The peptide bond is a resonance structure between two limiting states. Therefore the N-C bond has a partial double bond character and the atoms/groups C<sub>α</sub>, HN, C, C=O are within one plane. Peptide bonds are usually in '''trans''' conformation (ω torsion angle= 180°). In cis-conformation, there would be steric hindrance between C<sub>α,i</sub> and C<sub>α,i+1</sub>. | The peptide bond is a resonance structure between two limiting states. Therefore the N-C bond has a partial double bond character and the atoms/groups C<sub>α</sub>, HN, C, C=O are within one plane. Peptide bonds are usually in '''trans''' conformation (ω torsion angle= 180°). In cis-conformation, there would be steric hindrance between C<sub>α,i</sub> and C<sub>α,i+1</sub>. | ||
| - | [[Image:trans-cis-peptide-bond.png]] | + | [[Image:trans-cis-peptide-bond.png|200 px|text]] |
Cis conformation can occur only for a peptide bond preceding a Pro residue, see below. | Cis conformation can occur only for a peptide bond preceding a Pro residue, see below. | ||
Revision as of 15:21, 16 March 2010
Peptide bond
|
The (highlight in ) formation is a condensation reaction between the carboxyl group of the amino acid i and the amino group of the amino acid i+1.
The peptide bond is a resonance structure between two limiting states. Therefore the N-C bond has a partial double bond character and the atoms/groups Cα, HN, C, C=O are within one plane. Peptide bonds are usually in trans conformation (ω torsion angle= 180°). In cis-conformation, there would be steric hindrance between Cα,i and Cα,i+1.
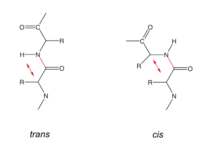
Cis conformation can occur only for a peptide bond preceding a Pro residue, see below.
is defined by the four atoms φ = C - N - Cα - C (in green).
is defined by the four atoms ψ = N - Cα - C - N (in green).
is defined by the four atoms ω = Cα - C - N - Cα (in green).
Cis peptide bonds
The ω torsion angle can adopt a value close to 0° (cis-conformation), when a Pro residue is the following residue (Xaa-Pro peptide bond). In this situation a and a are similarily unfavorable, since there is a steric clash between Cα,i with Cα,i+1 or Cδ,i+1, respectively. Conversely, the carbonyl O of residue i is in tight juxtaposition with Cδ,i+1 or Cα,i+1 (note that latter tight contact occurs in any trans peptide bond).
