Copper, Zinc Superoxide Dismutase
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
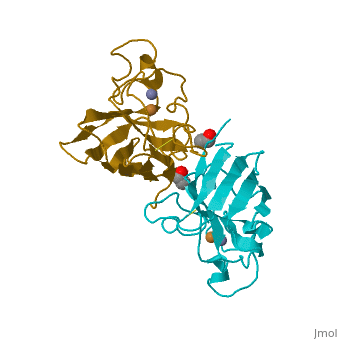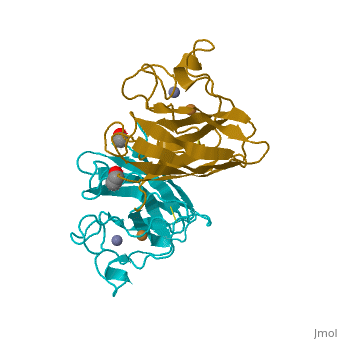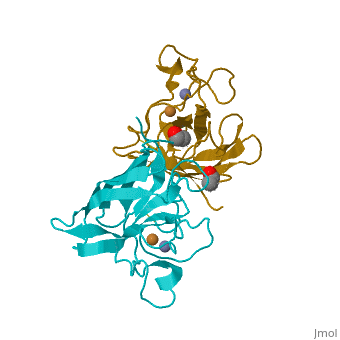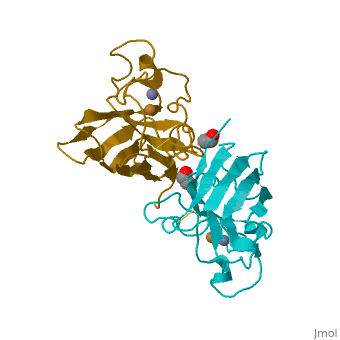
Cu/Zn Superoxide dismutase contains four polypeptide chains (B,O,G,Y). Each chain contains one alpha-helix and 12-14 beta-sheets. | Cu/Zn Superoxide dismutase contains four polypeptide chains (B,O,G,Y). Each chain contains one alpha-helix and 12-14 beta-sheets. | ||
| - | <applet load='2SOD' size='300' frame='true' align='right' caption=' | + | <applet load='2SOD' size='300' frame='true' align='right' caption='Cu/Zn Superoxide Dismutase' /> |
| - | [[Image:Example.jpg]] | ||
==References== | ==References== | ||
texttext<ref>PMID:#(specific#)</ref> | texttext<ref>PMID:#(specific#)</ref> | ||
Revision as of 01:40, 19 March 2010
Cu/Zn Superoxide Dismutase Cu/Zn Superoxide (SODc) dismutase is an oxidoreductase enzyme, catalyzing the dismutation of superoxide into oxygen and hydrogen peroxide.
Contents |
Introduction
Cu/Zn Superoxide dismutase is an important antioxidant defense in nearly all cells exposed to oxygen.
Cu2+ Zn Superoxide Dismutase belongs to the superfamily of oxidoreductases, specifically those acting on superoxide as acceptor.
The Reaction of Dismutase
General Structure
Cu/Zn Superoxide dismutase contains four polypeptide chains (B,O,G,Y). Each chain contains one alpha-helix and 12-14 beta-sheets.
|
References
texttext[1]
Proteopedia Page Contributors and Editors (what is this?)
Jordan Schibli, Jane S. Richardson, David Canner, Michal Harel, Andrea Gorrell