Copper, Zinc Superoxide Dismutase
From Proteopedia
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
Cu/Zn Superoxide dismutase is an important antioxidant defense in nearly all cells exposed to oxygen. | Cu/Zn Superoxide dismutase is an important antioxidant defense in nearly all cells exposed to oxygen. | ||
| - | |||
Cu2+ Zn Superoxide Dismutase belongs to the superfamily of oxidoreductases, specifically those acting on superoxide as acceptor. | Cu2+ Zn Superoxide Dismutase belongs to the superfamily of oxidoreductases, specifically those acting on superoxide as acceptor. | ||
| + | |||
| + | Dismutation is a term that refers to a special type of reaction, where two equal but opposite reactions occur on two separate molecules. SOD takes two molecules of superoxide, strips the extra electron off of one, and places it on the other. So, one ends up with an electron less, forming normal oxygen, and the other ends up with an extra electron. The one with the extra electron then rapidly picks up two hydrogen ions to form hydrogen peroxide. Of course, hydrogen peroxide is also a dangerous compound, so the cell must use the enzyme catalase to detoxify it. | ||
==The Reaction of Dismutase== | ==The Reaction of Dismutase== | ||
| + | 2 O2 -. + 2H+ SOD> 02 + H2O2 | ||
==General Structure== | ==General Structure== | ||
| Line 12: | Line 14: | ||
<applet load='2SOD' size='300' frame='true' align='right' caption='Cu/Zn Superoxide Dismutase' /> | <applet load='2SOD' size='300' frame='true' align='right' caption='Cu/Zn Superoxide Dismutase' /> | ||
| - | |||
| - | |||
==References== | ==References== | ||
texttext<ref>PMID:#(specific#)</ref> | texttext<ref>PMID:#(specific#)</ref> | ||
Revision as of 20:12, 19 March 2010
Cu/Zn Superoxide Dismutase Cu/Zn Superoxide (SODc) dismutase is an oxidoreductase enzyme, catalyzing the dismutation of superoxide into oxygen and hydrogen peroxide.
Contents |
Introduction
Cu/Zn Superoxide dismutase is an important antioxidant defense in nearly all cells exposed to oxygen. Cu2+ Zn Superoxide Dismutase belongs to the superfamily of oxidoreductases, specifically those acting on superoxide as acceptor.
Dismutation is a term that refers to a special type of reaction, where two equal but opposite reactions occur on two separate molecules. SOD takes two molecules of superoxide, strips the extra electron off of one, and places it on the other. So, one ends up with an electron less, forming normal oxygen, and the other ends up with an extra electron. The one with the extra electron then rapidly picks up two hydrogen ions to form hydrogen peroxide. Of course, hydrogen peroxide is also a dangerous compound, so the cell must use the enzyme catalase to detoxify it.
The Reaction of Dismutase
2 O2 -. + 2H+ SOD> 02 + H2O2
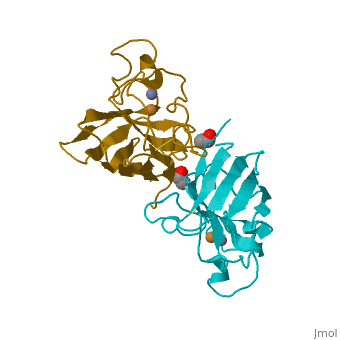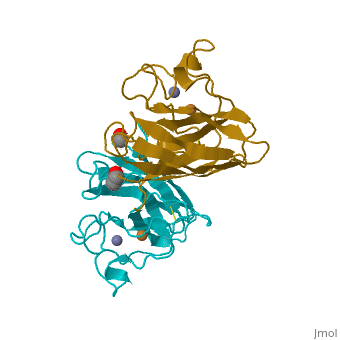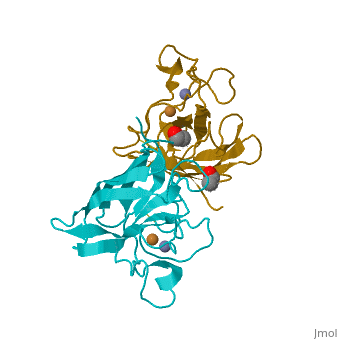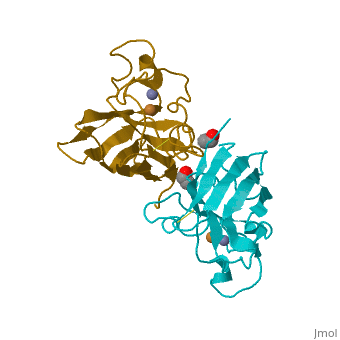
General Structure
Cu/Zn Superoxide dismutase contains four polypeptide chains (B,O,G,Y). Each chain contains one alpha-helix and 12-14 beta-sheets.
|
References
texttext[1]
Proteopedia Page Contributors and Editors (what is this?)
Jordan Schibli, Jane S. Richardson, David Canner, Michal Harel, Andrea Gorrell