We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 156
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <table style="background-color:#ffffc0" cellpadding="8" width="95%" border="0"><tr><td>Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. [[User:Andrea Gorrell|Andrea Gorrell]].</td></tr> | ||
| - | Anthony Daniele | ||
| - | |||
= Chloramphenicol Acetyltransferase Type III = | = Chloramphenicol Acetyltransferase Type III = | ||
| Line 21: | Line 18: | ||
==Structure== | ==Structure== | ||
<applet load='4CLA' size='300' frame='true' align='right' caption='' /> | <applet load='4CLA' size='300' frame='true' align='right' caption='' /> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <table style="background-color:#ffffc0" cellpadding="8" width="95%" border="0"><tr><td>Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. [[User:Andrea Gorrell|Andrea Gorrell]].</td></tr> | ||
Revision as of 04:45, 21 March 2010
Contents |
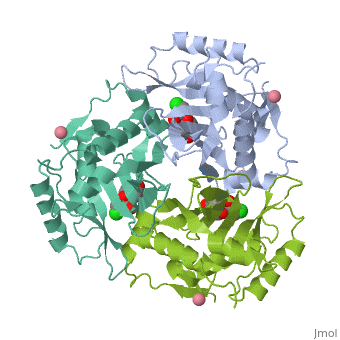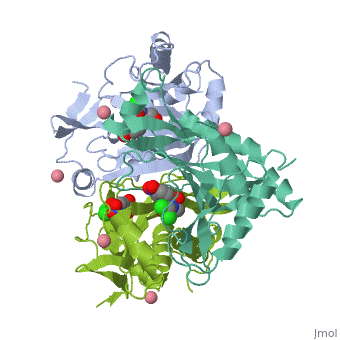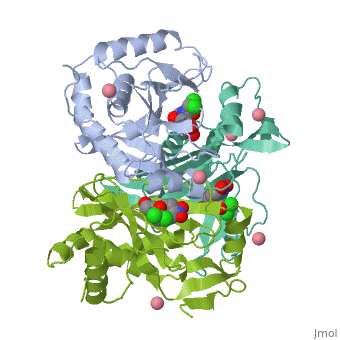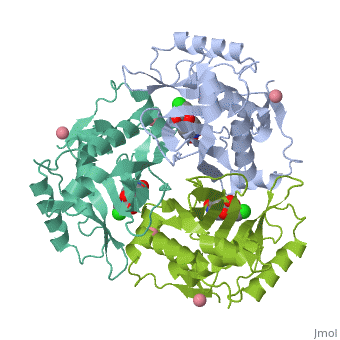
Chloramphenicol Acetyltransferase Type III
Chloramphenicol acetyltransferase type III (CAT III) is an enzyme which catalyzes the transfer of an acetyl group from acetyl-CoA to hydroxyl groups of chloramphenicol. CAT III is a trimeric protein
Introduction
Reaction of CAT III
In the first step of the reaction, Histidine-156 abstracts a proton from the 3-hydroxyl of chloramphenicol, promoting a nucleophilic attack from the deprotonated oxygen to the thioester bond of the acetyl-CoA. The intermediate produced, 3-acetylchloramphenicol, then rearranges non-enzymatically to 1-acetylchloramphenicol. Regeneration of the 3-hydroxyl allows another CAT III catalyzed nucleophilic attack to another acetyl-CoA and a 1,3-diacetylchloramphenicol product is formed.
Structure
|
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |