Ozonolysis
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
*We can also watch the <scene name='Ozonolysis/Ozonolysis_step_1_fix/6'>reaction (ball and stick)</scene>, or the | *We can also watch the <scene name='Ozonolysis/Ozonolysis_step_1_fix/6'>reaction (ball and stick)</scene>, or the | ||
{{Template:Button_Toggle_Animation2}} | {{Template:Button_Toggle_Animation2}} | ||
| - | *Reaction with <scene name='Ozonolysis/Ozonolysis_step_1_fix/ | + | *Reaction with <scene name='Ozonolysis/Ozonolysis_step_1_fix/8'>spacefilling atoms</scene>, or the |
*Reaction with translucent atoms. | *Reaction with translucent atoms. | ||
Revision as of 17:56, 21 March 2010
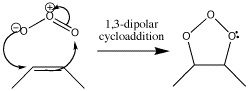
Ozonolysis is a type of cycloaddition which destroys bonds. It starts with a 1,3-dipolar cycloaddition but eventually becomes a method of cleaving π bonds in an oxidative fashion, so that they end up as two carbonyl groups. The reagent for this reaction is ozone, O3.
Reaction
|
- Here are the electron pushing .
- We can also watch the , or the
- Reaction with , or the
- Reaction with translucent atoms.
Acknowledgements
The animations of the ozonolysis reaction, as well as the 2D images of the reaction mechanism, were created by Nick Greeves. Many more reactions are viewable in an intuitive manner at http://www.chemtube3d.com.