Sandbox 160
From Proteopedia
| Line 24: | Line 24: | ||
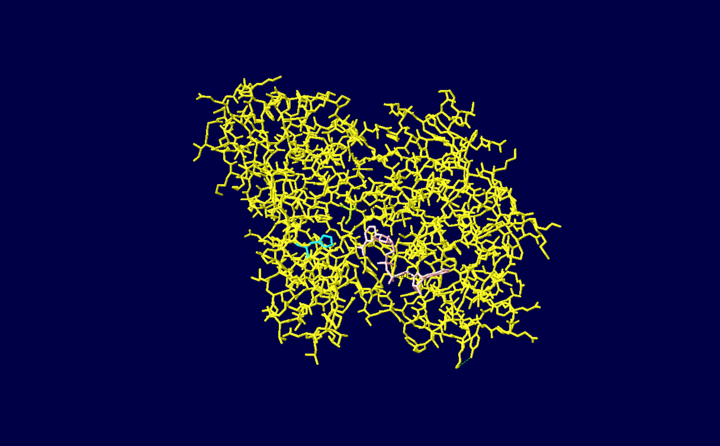
| - | [[Image:1vc2 image.png]] | + | [[Image:1vc2 image.png | thumb | upright=4.0 | Figure 1. Illustration created using PDB software highlighting the NAD+ ligand and Cysteine and Histidine residues within the active site of 1vc2.]] |
Revision as of 03:40, 24 March 2010
Introduction
| |||||||||
| 1vc2, resolution 2.60Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Activity: | Glyceraldehyde-3-phosphate dehydrogenase (phosphorylating), with EC number 1.2.1.12 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum, TOPSAN | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Glyceraldehyde 3-Phosphate dehydrogenase (GAPDH) is an Oxidoreductase enzyme and is involved in many important biochemical reactions. This protein is responsible for catalyzing the conversion of glyceraldeyde 3-Phosphate into 1,3-Biphosphoglycerate in a two step coupled mechanism. This conversion occurs during step 6 or the beginning of the "payoff phase" of glycolysis (the second half of the entire process) in which ATP and NADH is produced. A total of 2 NADH and 4 ATP are produced during this phase for a net gain of 2 NADH and 2 ATP for the entire glycolysis pathway per glucose.
Structure & Function
The enzyme contains a NAD+ group which functions as a hydrogen acceptor during the course of the reaction which is bound to a Rossman fold. During the catalysis of glyceraldehyde 3-phosphate to 1,3-biphosphoglycerate a hydride ion is enzymatically transferred from the aldehyde group of glyceraldehyde 3-phosphate to the nicotinamide ring of NAD+ reducing it to NADH (pink). The active site of GAPDH contains a cysteine (Cys149 colored green) residue which reacts with the glyceraldehyde 3-phosphate molecule through its -SH group. The substrate is covalently bound during the reaction through its aldehyde group to the -SH group of the cysteine residue and the resulting reaction produces a thiohemiacetal. Note that this reaction occurs through acid base catalysis with aid of a histidine residue (His176). The of the molecule is illustrated below.
A simplified illustration of the net reaction is as follows:
D-glyceraldehyde 3-phosphate + phosphate + NAD+ ---------> 1,3 biphospho-D-glycerate + NADH + H+
The Pi that is involved in the reaction functions to attack by phosphorolysis the thioester intermediate that is formed by the substrate on the cysteine reside after NAD+ has been reduced. The attack by Pi on the carbonyl carbon of C1 is simultaneously followed by the replacement of bound NADH for NAD+ so another turn of the cycle can now commence. The final product is released as 1,3 bisphosphoglycerate in which the second Pi molecule has been incorporated.
Active Site in Detail
Once Glyceraldehyde 3-phophate comes into contact with the active site it forms a hydrogen bond through its C2 hydroxyl group to Cys149N. The C1 hydroxyl group of the substrate binds to His176NE2. Additional hydrogen bonds to the phosphate group of the substrate from additional residues such as Thr1790G1, Arg231NH1(these two residues are not highlighted in the below illustration) along with N7N and 02'N of the NAD+ moeity (pink) help stabilize the molecule during the course of the reaction in the active site. The nicotinamide ring of the NAD+ ligand is responsible for orienting the hydrogen atom at C1 towards itself which allows for easier transfer in producing in reducing NAD+ to NADH.