Prolyl Endopeptidase
From Proteopedia
| Line 14: | Line 14: | ||
== Function == | == Function == | ||
| - | PEPs are thought to have a role in the degredation of neuropeptides due to the high concentration of PEPs in the brain and the fact that PEPs have been shown to degrade several neuropeptides(vasopressin, β-endorphin, thyroliberin). | + | PEPs are thought to have a role in the degredation of neuropeptides due to the high concentration of PEPs in the brain and the fact that PEPs have been shown to degrade several neuropeptides(vasopressin, β-endorphin, thyroliberin). The distribution of PEP in the brain has been found to be similar to that of certain receptors of neuropeptides which supports PEPs being involved in the degradation of neuropeptide transmitters. |
=== Binding Mechanism === | === Binding Mechanism === | ||
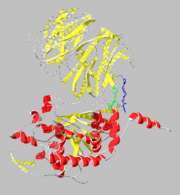
| - | [[Image: | + | [[Image:1yr2_ribbon2.png|thumb|alt=Alt text|Catalytic( and β-propeller domains(]] |
=== Inhibition === | === Inhibition === | ||
| Line 23: | Line 23: | ||
== Pharmaceutical Possibilities == | == Pharmaceutical Possibilities == | ||
| + | Both human and microbial PEP have been the focus of research into their viability as therapeutic agents for several diseases. The ability of PEPs to cleave internal proline peptide bonds is of interest in the treatment of Celiac Disease which is caused by a reaction to gluten, a proline rich protein found in wheat and wheat subspecies. CITE BESEDIN PEPs have also been linked to several neurological disorders due to high activity in the brain and proposed role in the degradation of neuropeptides. | ||
=== Celiac Disease === | === Celiac Disease === | ||
Revision as of 20:02, 25 March 2010
|
Prolyl endopeptidases (PEPs) are a class of serine proteases which cleave peptide bonds on the c-terminal side of internal proline residues.
Contents |
Structure
β-Propeller Domain
Catalytic Domain
Domain Interface
Function
PEPs are thought to have a role in the degredation of neuropeptides due to the high concentration of PEPs in the brain and the fact that PEPs have been shown to degrade several neuropeptides(vasopressin, β-endorphin, thyroliberin). The distribution of PEP in the brain has been found to be similar to that of certain receptors of neuropeptides which supports PEPs being involved in the degradation of neuropeptide transmitters.
Binding Mechanism
Inhibition
Pharmaceutical Possibilities
Both human and microbial PEP have been the focus of research into their viability as therapeutic agents for several diseases. The ability of PEPs to cleave internal proline peptide bonds is of interest in the treatment of Celiac Disease which is caused by a reaction to gluten, a proline rich protein found in wheat and wheat subspecies. CITE BESEDIN PEPs have also been linked to several neurological disorders due to high activity in the brain and proposed role in the degradation of neuropeptides.
Celiac Disease
Neurological Disorders
References
Proteopedia Page Contributors and Editors (what is this?)
Stacey Shantz, Michal Harel, Alexander Berchansky, Joel L. Sussman, Andrea Gorrell, David Canner