Copper, Zinc Superoxide Dismutase
From Proteopedia
| Line 32: | Line 32: | ||
Free radicals such as superoxide are a type of [http://en.wikipedia.org/wiki/Reactive_oxygen_species reactive oxygen species] that can strip electrons from proteins, lipids, or nucleic acids, thereby destroying their functions and resulting in cell dysfunction or death (2). Apparently, if SOD is defective, superoxide is not degraded and can destroy cells. Motor neurons appear to be particularly sensitive to superoxide attack. | Free radicals such as superoxide are a type of [http://en.wikipedia.org/wiki/Reactive_oxygen_species reactive oxygen species] that can strip electrons from proteins, lipids, or nucleic acids, thereby destroying their functions and resulting in cell dysfunction or death (2). Apparently, if SOD is defective, superoxide is not degraded and can destroy cells. Motor neurons appear to be particularly sensitive to superoxide attack. | ||
| - | [[Image:SOD1.png|thumb|Cu/Zn Superoxide dismutase polypeptide chain coloured by B-factors]] | + | [[Image:SOD1.png|thumb|left|Cu/Zn Superoxide dismutase polypeptide chain coloured by B-factors]] |
==Importance of Vitamins== | ==Importance of Vitamins== | ||
Revision as of 21:37, 25 March 2010
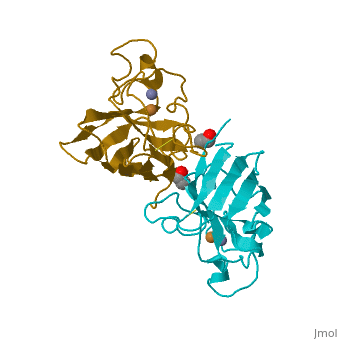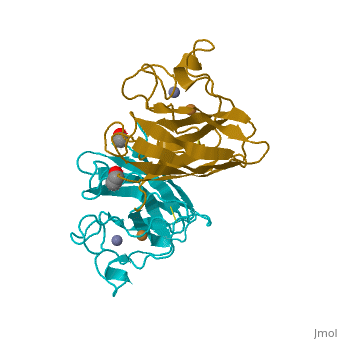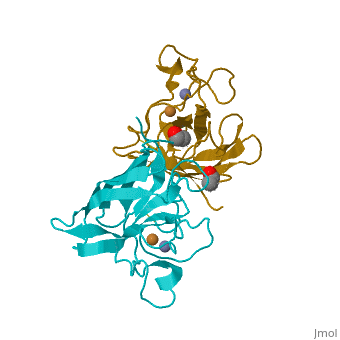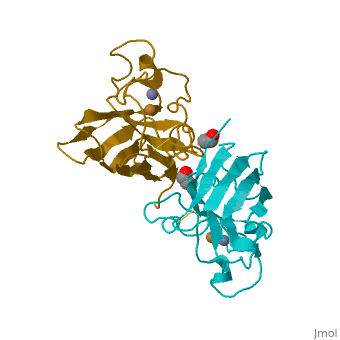
Cu/Zn Superoxide Dismutase Cu/Zn Superoxide (SODc) dismutase is an oxidoreductase enzyme, catalyzing the dismutation of superoxide into oxygen and hydrogen peroxide.
Contents |
Introduction
Oxygen is vital to sustain life; our cells cannot live without it. Oxygen is the final acceptor of electrons in respiration at the electron transport chain, allowing us to produce more energy from food. But oxygen is also a dangerous compound (1). Reactive forms of oxygen, such as superoxide, leak from the respiratory chain and wreak havoc on the cell. Superoxide is a free radical; radicals are molecules that readily accept electrons, which make them highly reactive. They can strip electrons from proteins, lipids, or nucleic acids, thereby destroying their functions and resulting in cell dysfunction or death. Free-radical damage has been implicated in Amyotrophic lateral sclerosis (ALS), Arteriosclerosis, Arthritis, Cancer, and Ageing(2). Superoxide dismutase is an enzyme that detoxifies superoxide via a special reaction known as dismutation.
Cu/Zn Superoxide dismutase is an important antioxidant defense in nearly all cells exposed to oxygen. Cu2+ Zn Superoxide Dismutase belongs to the superfamily of oxidoreductases, specifically those acting on superoxide as acceptor.
Superoxide Dismutase Family
In mammals there are three known isomers of superoxide dismutase (SOD). Copper and Zinc containing SOD1 is located in the cytoplasm. Manganese containing SOD2 is located in the mitochondria while a second Copper and Zinc containing SOD3 is located in the extracellular space. SOD3 and SOD2 are tetramers whereas SOD1 is a dimer. These enzymes perform the dismutation reaction by a similar mechanism.
The Reaction of Dismutase
Dismutation is a term that refers to a special type of reaction, where two equal but opposite reactions occur on two separate molecules (1).
Superoxide is produced both accidentally and also as a reactive oxygen species required for a number of enzyme-catalyzed reactions. Copper-Zinc superoxide dismutase catalyze the reaction between superoxide and water to yield oxygen and hydrogen peroxide.
Hydrogen peroxide is then scavenged by catalase, a ubiquitous heme protein that catalyzes the Dismutationof hydrogen peroxide into water and molecular oxygen (4).
<math>2H_2 O_2 → 〖2H〗_2 O + O_2 </math>
General Structure
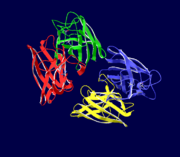
Cu/Zn Superoxide dismutase is a homotetramer, containing four polypeptide chains (B,O,G,Y), 152 residues in length. Each chain contains one alpha-helix and 12-14 beta-sheets. It has a structural weight of 62913.70. It has a ligand identifier of C2 H4 O
|
Antioxidant Capabilities
Free radicals such as superoxide are a type of reactive oxygen species that can strip electrons from proteins, lipids, or nucleic acids, thereby destroying their functions and resulting in cell dysfunction or death (2). Apparently, if SOD is defective, superoxide is not degraded and can destroy cells. Motor neurons appear to be particularly sensitive to superoxide attack.
Importance of Vitamins
Superoxide dismutase and catalase are remarkably efficiently, performing their reactions at or near the diffusion-limited rate. Glutathione peroxide also plays a role in scavenging H2O2. Other cellular defences against oxidative damage include the antioxidant vitamins, vitamins E and C. Because it is lipophilic, vitamin E is especially useful in protecting membranes from lipid peroxidation.
One of the long-term benefits of exercise may be to increase the amount of superoxide dismutase in the cell. The elevated aerobic metabolism during exercise causes more ROS to be generated. In response, the cell synthesizes more protective enzymes. The net effect is one of protection, because the increase in superoxide dismutase more effectively protects the cell during periods of rest.
See Also
Free-Radical Theory of Aging (FRTA)
References
texttext[1] (1)
Seeley, Stephens, Tate. (2008). Anatomy & Physiology 8th Ed. (2)
RCSB
Free Radicals and Antioxidant Nutrients ch. 6 pg. 484
Proteopedia Page Contributors and Editors (what is this?)
Jordan Schibli, Jane S. Richardson, David Canner, Michal Harel, Andrea Gorrell