Sandbox 177
From Proteopedia
| Line 1: | Line 1: | ||
== '''NADPH-cytochrome P450 oxidoreductase''' == | == '''NADPH-cytochrome P450 oxidoreductase''' == | ||
| + | <applet load='3es9' size='270' color='black' frame='true' align='right' scene='Sandbox_177/Jmol3es9/1' caption='NADPH-cytochrome P450 oxidoreductase' /> | ||
Taya O'Neill | Taya O'Neill | ||
==General Information== | ==General Information== | ||
| - | <applet load='3es9' size='300' color='black' frame='true' align='right' scene='Sandbox_177/Jmol3es9/1' caption='NADPH-cytochrome P450 oxidoreductase' /> | ||
| - | |||
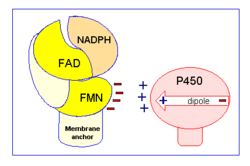
NADPH-cytochrome P450 oxidoreductase (CYPOR) is a ~78kDa, multidomain flavoprotein. <ref name="5TSON">PMID:19171935</ref> Containing three co-factors, FMN, FAD and NADPH, CYPOR is the archetype for the mammalian diflavin-containing enzyme family.<ref name="5TSON"/> | NADPH-cytochrome P450 oxidoreductase (CYPOR) is a ~78kDa, multidomain flavoprotein. <ref name="5TSON">PMID:19171935</ref> Containing three co-factors, FMN, FAD and NADPH, CYPOR is the archetype for the mammalian diflavin-containing enzyme family.<ref name="5TSON"/> | ||
Revision as of 01:00, 26 March 2010
Contents |
NADPH-cytochrome P450 oxidoreductase
|
Taya O'Neill
General Information
NADPH-cytochrome P450 oxidoreductase (CYPOR) is a ~78kDa, multidomain flavoprotein. [1] Containing three co-factors, FMN, FAD and NADPH, CYPOR is the archetype for the mammalian diflavin-containing enzyme family.[1]
Horecker first identified this protein in 1950 as NADPH-specific cytochrome c reductase, based on his assumption that it was the redox partner for cytochrome c, found in the mitochondria.[2] However, studies in the 1960s and later showed that its main function is actually as the redox partner for cytochrome P450 in microsomal electron transport chains, thus the name change (4).[1]
Regulation of this protein, which is found all tissues to some extent, is largely at the transcriptional level.[3] The thyroid hormone T3 in most cases, while adrenocorticotrophic hormone acts as a regulator in a few specific cases.[4][5]
Structure
CYPOR is a multidomain protein. The N-terminus consists of a single alpha-helix that functions as a transmembrane anchor (~6kDa), holding the protein in the endoplasmic reticulum. The portion of the protein responsible for reducing cytochrome P450 is soluble and ~66kDa. The first 170 residues of the soluble region are very similar to those of flavodoxin, which correlates to the fact that this is the area that binds FMN. FAD and NADPH binding domains are somewhat grouped together and located closer to the C-terminus. Between the FMN and FAD/NADPH bind domains is a connecting domain, which is a random coil and highly flexible. This section is presumed to be responsible for the relatively increased mobility of the FMN domain, changes to conformation and the relative orientation of the binding domains. Because of this, it plays a key role in the transfer of electrons between FMN and FAD. [1]
Function
In vivo CYPOR is believe to alternate between a one and a three electron reduced form. While the 1 electron form is fairly stable, forming a neutral blue semiquinone, it is the hydroquinone, or 3 electron form, that is able to donate electrons to the desired redox partners.
As part of the microsomal electron transport system, CYPOR moves electrons from NADPH -> FAD -> FMN -> cytochrome P450. Specifically a hydride anion is moved from NADPH to the FAD. The two electrons are then individually passed to FMN, in a process that is believed to be conformationally gated, before being passed on to cytochrome P450, again one at a time.[1] This reduction of cytochrome P450 allows it to function in biosynthesis and biodegradation pathways of a variety of endogenous and foreign hydrophobic substrates, including drugs and steroids.[1][6]
Cytochrome b5, cytochrome c and heme oxygenase can also receive electrons from CYPOR.[1] In these cases CYPOR is functioning in the heme degradation pathway, or with monooxygenase and/or 7-dehydrocholesterol reductase in sterol synthesis.[1]
A function that is of particular interest currently is CYPOR’s ability to activate anticancer prodrugs reductively.[1] This makes it a potential target for anticancer research and therapy.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Hamdane D, Xia C, Im SC, Zhang H, Kim JJ, Waskell L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J Biol Chem. 2009 Apr 24;284(17):11374-84. Epub 2009 Jan 26. PMID:19171935 doi:10.1074/jbc.M807868200
- ↑ Horecker BL. Triphosphopyridine nucleotide-cytochrome c reductase in liver. J Biol Chem 1950 April 1;183(2):593-605
- ↑ Li HC, Liu D, Waxman DJ. Transcriptional induction of hepatic NADPH: cytochrome P450 oxidoreductase by thyroid hormone. Mol Pharmacol. 2001 May;59(5):987-95. PMID:11306680
- ↑ Waxman DJ, Morrissey JJ, Leblanc GA. Hypophysectomy differentially alters P-450 protein levels and enzyme activities in rat liver: pituitary control of hepatic NADPH cytochrome P-450 reductase. Mol Pharmacol. 1989 Apr;35(4):519-25. PMID:2495435
- ↑ Ram PA, Waxman DJ. Thyroid hormone stimulation of NADPH P450 reductase expression in liver and extrahepatic tissues. Regulation by multiple mechanisms. J Biol Chem. 1992 Feb 15;267(5):3294-301. PMID:1737785
- ↑ Hasemann CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J. Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure. 1995 Jan 15;3(1):41-62. PMID:7743131
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |