Horse Liver Alcohol Dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== General Information== | == General Information== | ||
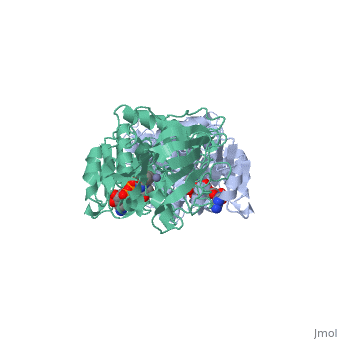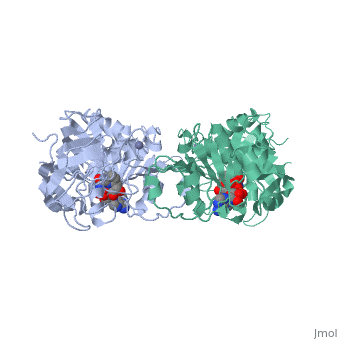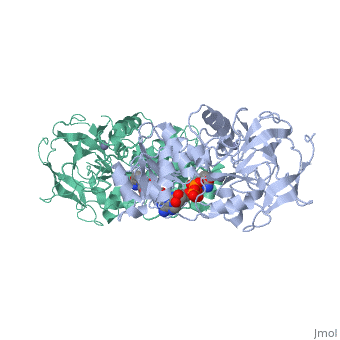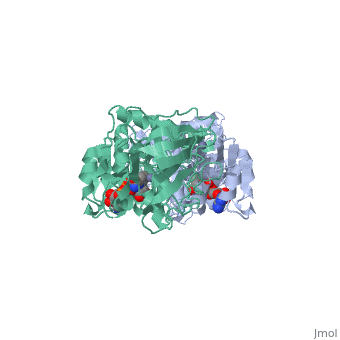
Alcohol dehydrogenase (LADH) is found in the liver of the species Equus Caballus (horse). It is a dimeric zinc-dependant protein, that catalyzes the reversible oxidation of primary and secondary alcohols to aldehyde, requiring the transfer of a hydride ion from the alcohol substrate to the cofactor nicotinamide adenine dinucleotide (NAD). | Alcohol dehydrogenase (LADH) is found in the liver of the species Equus Caballus (horse). It is a dimeric zinc-dependant protein, that catalyzes the reversible oxidation of primary and secondary alcohols to aldehyde, requiring the transfer of a hydride ion from the alcohol substrate to the cofactor nicotinamide adenine dinucleotide (NAD). | ||
| + | |||
| + | <applet load='1a72' size='300' frame='true' align='right' caption='Horse Liver Alcohol Dehydrogenase (LADH)' /> | ||
== Protein Structure == | == Protein Structure == | ||
Revision as of 01:41, 26 March 2010
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |
Contents |
Horse Liver Alcohol Dehydrogenase
Meghan Hatcher
General Information
Alcohol dehydrogenase (LADH) is found in the liver of the species Equus Caballus (horse). It is a dimeric zinc-dependant protein, that catalyzes the reversible oxidation of primary and secondary alcohols to aldehyde, requiring the transfer of a hydride ion from the alcohol substrate to the cofactor nicotinamide adenine dinucleotide (NAD).
|
Protein Structure
Protein Function
References
Proteopedia Page Contributors and Editors (what is this?)
Meghan Hatcher, Alexander Berchansky, David Canner, Michal Harel, Simmi Parhar, Andrea Gorrell, Eran Hodis