Pilin
From Proteopedia
| Line 9: | Line 9: | ||
==Structure== | ==Structure== | ||
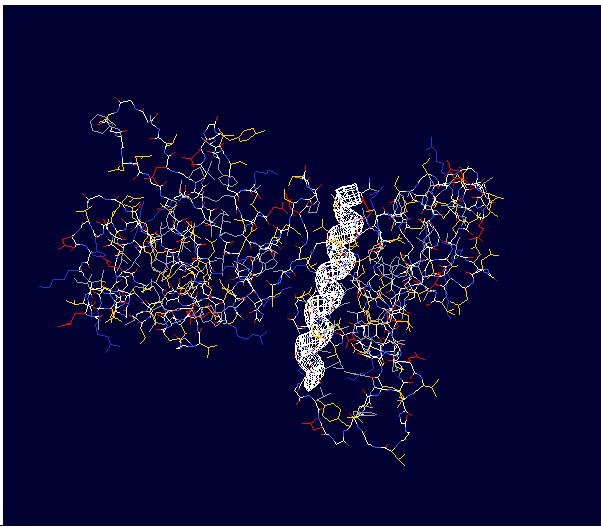
| - | Type IV pilin are homopolymers, composed of a single-chain pilin protein. A conserved 25 residue hydrophobic N terminal sequence is shared by all type IV pilins, as well as conserved pilus assembly machinery, and a unique N-methylated N-terminus. Type IVb pilins also contain a pair of conserved cysteines that form an intrachain disulphide bond<ref name=journal1>PMID:19626704</ref>. The cysteine-containing region in the C-terminus is thought to play function via the formation of disulfides in CFTR adhesion and in the assembly of PilS to make the pilus of the bacterium. The highly conserved N-terminal hydrophobic tail functions as a oligomerization domain for fibre formation | + | Type IV pilin are homopolymers, composed of a single-chain pilin protein. A conserved 25 residue hydrophobic N terminal sequence is shared by all type IV pilins, as well as conserved pilus assembly machinery, and a unique N-methylated N-terminus. Type IVb pilins also contain a pair of conserved cysteines that form an intrachain disulphide bond <ref name=journal1>PMID:19626704</ref>. |
| - | + | The type IVb pilus operon of ''S. typhi'' contains the piLS gene, which encodes a structural pilin. The cysteine-containing region in the C-terminus is thought to play function via the formation of disulfides in CFTR adhesion and in the assembly of PilS to make the pilus of the bacterium. The highly conserved N-terminal hydrophobic tail functions as a oligomerization domain for fibre formation <ref name=journal2>Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. | |
| + | Different protein folds for the type IVb pilins have been identified Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026</ref>. | ||
[[Image:N_Teeerminal_Hydrophobic.png|alt text]] | [[Image:N_Teeerminal_Hydrophobic.png|alt text]] | ||
Revision as of 01:48, 26 March 2010
Contents |
Salmonella typhi type IVb pilin (PilS)
Introduction
Type IV pili play an important role in the pathogenesis of many bacterial species; they are a required component for the adhesion of bacteria to their target cells. For example, Type IV pili are required for infection by the pathogens that cause cholera, typhoid, pneumonia, gonorrhea, and meningitis [1]. They may also mediate transformation, modulate target-cell specificity and play a role in twitching motility [2].
Structure
Type IV pilin are homopolymers, composed of a single-chain pilin protein. A conserved 25 residue hydrophobic N terminal sequence is shared by all type IV pilins, as well as conserved pilus assembly machinery, and a unique N-methylated N-terminus. Type IVb pilins also contain a pair of conserved cysteines that form an intrachain disulphide bond [1]. The type IVb pilus operon of S. typhi contains the piLS gene, which encodes a structural pilin. The cysteine-containing region in the C-terminus is thought to play function via the formation of disulfides in CFTR adhesion and in the assembly of PilS to make the pilus of the bacterium. The highly conserved N-terminal hydrophobic tail functions as a oligomerization domain for fibre formation [2].
Function
Type IV pili are diverse in function; they mediate numerous cellular functions via their polymeric machinery. These functions include cell adhesion, signaling, phage attachment, surface motility, biofilm formation and DNA uptake by natural transformation. Specifically, the type IVb pilus of Salmonella typhi is the adhesion factor that allows the pathogen to enter into the gastrointestinal cells of animals [1]. The target receptor for the S. typhi pilus is a sequence of ten residues from the first extracellular domain of cystic fibrosis transmembrane conductance regulator [2].
References
- ↑ 1.0 1.1 1.2 Balakrishna AM, Saxena AM, Mok HY, Swaminathan K. Structural basis of typhoid: Salmonella typhi type IVb pilin (PilS) and cystic fibrosis transmembrane conductance regulator interaction. Proteins. 2009 Nov 1;77(2):253-61. PMID:19626704 doi:10.1002/prot.22500
- ↑ 2.0 2.1 2.2 Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Elyse Yaremco, David Canner, Andrea Gorrell, Alexander Berchansky