UDP-galactopyranose mutase
From Proteopedia
| Line 1: | Line 1: | ||
| + | =UDP-galactopryranose= | ||
| + | {{STRUCTURE_3int | PDB=3int | SCENE= }} | ||
| + | Galactopyranose mutase, UDP-D-Galactopyranose furanomutase<ref name="BEO1">http://www.brenda-enzymes.org/php/result_flat.php4?ecno=5.4.99.9</ref> or flavoenzyme uridine 5′-diphosphate galactopyranose mutase (UGM)<ref Name="GWF2">Gruber TD, Westler WM, Kiessling LL, Forest KT. X-ray Crystallography Reveals a Reduced Substrate Complex of UDP-Galactopyranose Mutase Poised for Covalent Catalysis by Flavin. Biochemistry. 2009 Oct 6; 48(39): 9171-73. [http://www.ncbi.nlm.nih.gov/pubmed/19719175 PMID:19719175]</ref>. | ||
| + | It has a [http://www.cas.org/expertise/cascontent/registry/regsys.html registry number] of EC 5.4.99.9<ref name="BEO1"/><ref name="MFD3">http://www.mondofacto.com/facts/dictionary?UDP-galactopyranose+mutase</ref>; and is an [http://chemistry.about.com/od/chemistryglossary/a/isomerdef.htm isomerase], (L) [http://www.thefreedictionary.com/polypeptide polypeptide] that consists of a [http://www.answers.com/topic/dimer dimer] made up of 2 [http://dictionary.reference.com/browse/monomer monomers], a [http://medical-dictionary.thefreedictionary.com/homodimer homodimer]<ref name="PPE4">[http://www.pdb.org/pdb/explore/explore.do?structureId=3INT 3int RCSB PDB]</ref><ref name="BLB5">Beis K, Srikannathasan V, Liu H, Fullerton SWB, Bamford VA, Sanders DAR, Whitfield C, McNeil MR, Naismith JH. Crystal Structures of Mycobacteria tuberculosis and Klebsiella pneumoniae UPD-Galactopyranose Mutase in the Oxidized State and Klebsiella pneumoniae UPD-Galactopyranose Mutase in the (Active) Reduced State. J. Mol. Biol. 2005 May 13; 384(4): 971-982[http://www.ncbi.nlm.nih.gov/pubmed/15843027 PMID:15843027]</ref><ref name="GWK6">Gruber TD, Borrok MJ, Westler WM, Forest KT, Kiessling LL. Ligand Binding and Substrate Discrimination by UDP-Galactopyranose Mutase. J. Mol. Biol. 2009 Aug 14; 391(2): 327-340. [http://www.ncbi.nlm.nih.gov/pubmed/19500588 PMID:19500588]</ref>. | ||
| + | |||
| + | Each monomer consists of a single chain of 390 [http://encyclopedia.farlex.com/Amino+acid+residue amino acid residues], and 2 [http://en.wikipedia.org/wiki/Protein_domain domains] each: a GLF (UDP-galactopyronase mutase family) and a DAO (FAD dependent oxidoreductase domain)<ref name="PPE4"/><ref name="BLB5"/><ref name="GWK6"/><ref name="GWF2"/>. | ||
| + | Each chain is 33% [http://www.moleculesinmotion.com/protarch/page_helix/menu.html helical] (17 helices; 131 residues) and 20% [http://www.eurekalert.org/multimedia/pub/6910.php?from=109258 beta sheet] (19strands; 81 residues)<ref name="PPE4"/>. | ||
| + | |||
| + | UDP-galactopryranose mutase consists of the genes glf and rfbd isolated from [http://emedicine.medscape.com/article/219907-overview ''Klebisiella pneumoniae''] (stain 01 (ATCC 13882)) and [http://www.microbiologybytes.com/video/Ecoli.html ''Escherichia coli''] (stain BI21(de3)) through a [http://www.accessexcellence.org/RC/VL/GG/inserting.php plasma vector]<ref name="PPE4"/>; can also be found in [http://www.microbiologybytes.com/video/Mtuberculosis.html ''Mycobacteria tuberculosis'']<ref name="GWF2"/><ref name="BLB5"/><ref name="GWK6"/><ref name="ZLH7">Zhang Q, Lui HW. Studies of UDP-Galactopyranose Mutase from Escherichia coli: An Unusual Role of Reduced FAD in its Cataysis. J. Am. Chem. Soc. 2000 Sep 27;122(38): 9065-70. [http://pubs.acs.org/doi/abs/10.1021/ja001333z DOI: 10.1021/ja001333z]</ref><ref name="YBY8">Yao X, Bleile DW, Yuan Y, Chao J, Sarathy KP, Sanders DAR, Pinto BM, O’Neill MA. Substrate Directs Enzyme Dynamics by Bridging Distal Sites: UPD-Galactopyranose Mutase. Proteins: Structure, Function, Bioinformatics. 2008 June 30; 74(4): 972-79. [http://www.ncbi.nlm.nih.gov/pubmed/18767162 PMID:18767162]</ref>. | ||
| + | |||
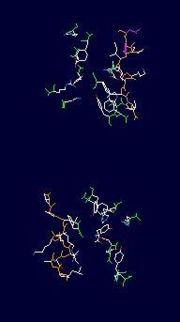
| + | this is the a close up view of the of one <scene name='152/Monomer_ative_sites/1'>active sites</scene> of one of the monomers that make up UDP-galactopryranose mutase. | ||
| + | |||
| + | ---- | ||
| + | ==History:== | ||
| + | The structure of the enzyme, UDP-galactopryranose mutase, was first solved by [http://www.webmineral.com/help/XRayDiffraction.shtml X-ray diffraction], at a resolution of 2.51Å, by a crystalline structure up tained through [http://www.bio.davidson.edu/courses/MolBio/MolStudents/spring2003/Kogoy/protein.html vapour diffusion] by a [http://www.bio.davidson.edu/courses/MolBio/MolStudents/spring2003/Kogoy/protein.html hanging drop] process, at a [http://www.elmhurst.edu/~chm/vchembook/184ph.html pH] of 5.6 and temperature of 289.0[http://www.windows.ucar.edu/cgi-bin/tour_def/earth/Atmosphere/temperature/kelvin.html K]<ref name="PPE4"/>. | ||
| + | The [http://encyclopedia.farlex.com/Enzyme-substrate+complex enzyme-substrate complex] structure was first crystallized to a 2.5Å resolution using the [http://www.tcm.phy.cam.ac.uk/~mds21/thesis/node51.html substrate analogue] UDP-glucose [http://en.wikipedia.org/wiki/Uridine_diphosphate_glucose (UDP-Glc)] that binds the same at the same active site as the native substrate but does not react to give product due to having an equatorial C4-OH group rather than an axial one<ref name="GWK6"/>; meaning that the UPD-Glc’s C4-OH group can not engage in a [http://www.historyoftheuniverse.com/hydrbond.html hydrogen bond] with C4 carbonyl of the reduced flavin cofactor that the native substrate’s, Galactose’s, C4-Oh group can<ref name="GWF2"/>. | ||
| + | |||
| + | Structures related to, similar to, the structure of UDP-galactopryranose (3int) include those of: [http://www.pdb.org/pdb/explore/explore.do?structureId=3INT I8T, 1V0J, 1WAM, 2BI7, 2BI8, 3GF4, 3INR]<ref name="PPE4"/>. | ||
| + | |||
| + | |||
| + | |||
| + | ---- | ||
| + | ==Reaction:== | ||
| + | UDP-galactopryranose mutase [http://www.biology-online.org/dictionary/Catalyses catalyses] the interconvertion of UPD-Galactopyranose, UDP-D-Galactopyranose, [http://en.wikipedia.org/wiki/UDP-galactopyranose_mutase (UDP-Gal''p'')] to, UDP D Galacto-1-4-Furanose, [http://biocyc.org/META/NEW-IMAGE?type=COMPOUND&object=UDP-GALACTOSE (UPD-Gal''f'')] with the help of noncovalentely bound, reduced [http://www.thefreedictionary.com/flavoprotein flavoprotien], (dihydro)flavin adenine dinucleotide [http://en.wikipedia.org/wiki/FAD (FAD)]<ref name="BEO1"/><ref name="GWF2"/><ref name="MFD3"/><ref name="PPE4"/><ref name="BLB5"/><ref name="GWK6"/><ref name="ZLH7"/><ref name="YBY8"/>with galactose-uridine-5’-diphosphate and uridine-5’-diphophate<ref name="PPE4"/>. | ||
| + | [http://en.wikipedia.org/wiki/Redox Reduction] of the FAD involves a transformation of the [http://www.britannica.com/EBchecked/topic/124242/coenzyme coenzyme] from a highly conjugated planar frame to a bent butterfly structure, which induces a [http://www.biology-online.org/dictionary/Conformational_change conformational change] within the enzyme making in more conductive to catalysis, and increases activity rate<ref name="GWF2"/><ref name="ZLH7"/>. This flavin reduction also results in the translocation of the mobile loop inward toward the substrate with the Uridine portion of the ligand moving upward toward the flavin<ref name="GWF2"/>. The flavin reduction and corresponding conformational change results in the enzyme being primed for covalent catalysis<ref name="GWF2"/>. | ||
| + | |||
| + | [[Image:Mech2.JPG |thumb|center|upright=2.0|The inter-conversion of UDP-Gal''p'' and UPD-Gal''f'' catalyzed by UGM, through a nucleophilic S<sub>N</sub>1 or S<sub>N</sub>2 attack by reduced FAD <ref name="GWK6"/>]] | ||
| + | |||
| + | The conversion between UDP-Gal''p'' and UPD-Gal''f'' involves the cleavage of the [http://www.answers.com/topic/anomer anomeric] C-O bond, the reaction involves distortion of the ring allowing access to the O4 on C-1 to release the UDP<ref name="GWF2"/><ref name="ZLH7"/>. | ||
| + | |||
| + | There is a covalent adduct created between the flavin and the substrate that involves a [http://medical-dictionary.thefreedictionary.com/Nucleophilic+attack nucleophilic attack] (S<sub>N</sub>1/2) by the nitrogen atom (N5) of the isoalloxazine ring on the substrate which creates covalent falvin-galacrose [http://dictionary.reference.com/browse/adduct adduct], that affords the formation of an iminium intermediate in the mechanism, and a displaces the UPD, as a good leaving group, which in turn allows the galactose ring to attach to the flavin, rearrange, dissociate and give the final product<ref name="GWF2"/><ref name="BLB5"/><ref name="GWK6"/>. | ||
| + | The C4 of the flavin provides a means to shuttle protons from the C4-OH to the nascent C5-OH after ring opening<ref name="GWF2"/>. | ||
| + | |||
| + | The substrate-binding, whose site is adjacent to the flavin cofactor directs the dynamic bridging of a recognition loop with distal FAD, which works to close the recognition loop over the substrate in the active site, and W160 sites, which interact with the Uralic of the substrate and is the N-terminal anchor of the recognition loop<ref name="GWK6"/><ref name="YBY8"/>. | ||
| + | |||
| + | Arg174, located outside the putative active site on a mobile loop (recognition loop) adjacent to the putative active site pointing away from the flavin; once the substrate binds it closes this loop and brings the Arg174 side chain in toward the pyrophosphoryl group of the ligand<ref name="GWK6"/>. This ‘recognition’ loop closes over the substrate-binding site, and is there by ‘locked’ down by the Arg174 coordination of the α-phosphate<ref name="GWF2"/><ref name="YBY8"/>. | ||
| + | |||
| + | |||
| + | ---- | ||
| + | ==Binding sites and important residues:== | ||
| + | [[Image:Ribbon import.JPG|thumb|left|(A)This is an image of the important residues involved in binding in ribbon form; the orange is the recognition loop, while the green are various other residues described in the text to the right.]] | ||
| + | [[Image:Sidechain import.JPG|thumb|right|(B)This is an image of the important residues' sidechains involved in binding in stick form; the slighlty orange backbone is the recognition loop, while the slightly green backbones are various other residues described in the text to the left.]] | ||
| + | The cleft to which the [http://en.wikipedia.org/wiki/FAD flavin] binds, in each monomer, is, adjacent to the [http://en.wikipedia.org/wiki/Active_site substrate binding site] and is, lined with conserved residues 167-177: His63, Tyr155, Gln159, Trp160, Try185, Phe186, Arg250, Asn270, Arg280, Tyr314 and Asp351<ref name="GWF2"/><ref name="BLB5"/><ref name="GWK6"/>. These, collectively, have a charge density consistent with binding of a negatively charged [http://dictionary.reference.com/browse/substrate substrate] such as reduced FAD<ref name="BLB5"/><ref name="GWK6"/><ref name="YBY8"/>. | ||
| + | |||
| + | Other residues involved in the conformational change of UGM, once the substrate is bound, are: Phe152, which sandwiches the Uralic ring of the [http://www.answers.com/topic/ligand ligand] between it’s [http://medical-dictionary.thefreedictionary.com/aromatic+ring aromatic ring] and that of Tyr155, and Leu175, which is located on the mobile loop moves to be adjacent to the bridging O of the ribose moiety and Phe152<ref name="GWK6"/>. | ||
| + | |||
| + | To position the sugar moiety the flavin, Tyr349, Arg280, Asn84, and an ordered water molecule all work together while theArg174 coordinates the α and β phosphates of the UDP, and with Try349 stabilizes the closed loop ‘locking’ it down<ref name="GWF2"/><ref name="YBY8"/>. | ||
| + | |||
| + | Arg280 also works to coordinate the α and β phosphates<ref name="GWK6"/><ref name="YBY8"/>. Arg280 is located in the flavin-binding site<ref name="GWK6"/>. | ||
| + | |||
| + | This closing of the loop involves residues 167-177 which are adjacent to the Uridine diphosphoryl portion if the ligand and involves a extension of a short helix composed of residues 169-171 encompassing residues 72-174 moving Arg174 in toward the active site<ref name="GWF2"/><ref name="GWK6"/>. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ---- | ||
| + | ==Also:== | ||
| + | The understanding of the structure of UDP-galactopryranose mutase and the mechanism with which it binds its substrates and catalyzes its reaction is of importance to [http://en.wikipedia.org/wiki/Pharmaceutical_drug pharmaceutical drug therapy] because UPD-Galactosefuranose and UPD-Galactopyranose are found in many [http://www.medterms.com/script/main/art.asp?articlekey=6383 pathogens], in their surface constituents, [http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1517-83822008000200001 cell wall glycoconjugates] and in a vital component of [http://en.wikipedia.org/wiki/Arabinogalactan arabinogalactan] that connects [http://www.biology-online.org/dictionary/Peptidoglycan peptidoglycan] and [http://www.cyberlipid.org/fa/acid0006.htm mycolic acids] in myobacteria cell walls in the lipoplysaccaride (LPS) [http://www.online-medical-dictionary.org/O+Antigens.asp?q=O+Antigens O antigens] of some [http://www.buzzle.com/articles/gram-negative-bacteria.html Gram-negative] bacteria; but are not found in human/mammal tissues so the this enzyme, UDP-galactopryranose mutase, that interconverts them can be safely [http://www.merriam-webster.com/dictionary/inhibition inhibited] slowing and preventing the growth of pathogenic microbes such as ''Escherichia coli'', ''Mycobacteria tuberculosis'', or ''Klebsiella pneumoniae''<ref name="GWF2"/><ref name="PPE4"/><ref name="BLB5"/><ref name="GWK6"/><ref name="ZLH7"/><ref name="YBY8"/>. | ||
| + | |||
| + | |||
| + | ---- | ||
| + | ==References:== | ||
| + | <references/> | ||
| + | ---- | ||
| + | ==Images:== | ||
| + | Images with in the Jmol applet were provided and created by protopedia; the inital view (the defaultt) has provided by protopedia and the green link with in the text (the active site) was created using Jmol within the protopedia applet edit function. | ||
| + | |||
| + | Images (A and B) of protein ribbon portions and important side chains were created using the [http://www.pdb.org/pdb/explore/explore.do?structureId=3INT 3int PDB] file from the [http://www.pdb.org/pdb/home/home.do RCSB] Bank in the [http://spdbv.vital-it.ch/ Swiss PDB Deep-Viewer]. | ||
| + | ---- | ||
<table style="background-color:#ffffc0" cellpadding="8" width="95%" border="0"><tr><td>Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. [[User:Andrea Gorrell|Andrea Gorrell]].</td></tr> | <table style="background-color:#ffffc0" cellpadding="8" width="95%" border="0"><tr><td>Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. [[User:Andrea Gorrell|Andrea Gorrell]].</td></tr> | ||
Christine Brown | Christine Brown | ||
Revision as of 21:34, 30 March 2010
Contents |
UDP-galactopryranose
Template:STRUCTURE 3int Galactopyranose mutase, UDP-D-Galactopyranose furanomutase[1] or flavoenzyme uridine 5′-diphosphate galactopyranose mutase (UGM)[2]. It has a registry number of EC 5.4.99.9[1][3]; and is an isomerase, (L) polypeptide that consists of a dimer made up of 2 monomers, a homodimer[4][5][6].
Each monomer consists of a single chain of 390 amino acid residues, and 2 domains each: a GLF (UDP-galactopyronase mutase family) and a DAO (FAD dependent oxidoreductase domain)[4][5][6][2]. Each chain is 33% helical (17 helices; 131 residues) and 20% beta sheet (19strands; 81 residues)[4].
UDP-galactopryranose mutase consists of the genes glf and rfbd isolated from Klebisiella pneumoniae (stain 01 (ATCC 13882)) and Escherichia coli (stain BI21(de3)) through a plasma vector[4]; can also be found in Mycobacteria tuberculosis[2][5][6][7][8].
this is the a close up view of the of one of one of the monomers that make up UDP-galactopryranose mutase.
History:
The structure of the enzyme, UDP-galactopryranose mutase, was first solved by X-ray diffraction, at a resolution of 2.51Å, by a crystalline structure up tained through vapour diffusion by a hanging drop process, at a pH of 5.6 and temperature of 289.0K[4]. The enzyme-substrate complex structure was first crystallized to a 2.5Å resolution using the substrate analogue UDP-glucose (UDP-Glc) that binds the same at the same active site as the native substrate but does not react to give product due to having an equatorial C4-OH group rather than an axial one[6]; meaning that the UPD-Glc’s C4-OH group can not engage in a hydrogen bond with C4 carbonyl of the reduced flavin cofactor that the native substrate’s, Galactose’s, C4-Oh group can[2].
Structures related to, similar to, the structure of UDP-galactopryranose (3int) include those of: I8T, 1V0J, 1WAM, 2BI7, 2BI8, 3GF4, 3INR[4].
Reaction:
UDP-galactopryranose mutase catalyses the interconvertion of UPD-Galactopyranose, UDP-D-Galactopyranose, (UDP-Galp) to, UDP D Galacto-1-4-Furanose, (UPD-Galf) with the help of noncovalentely bound, reduced flavoprotien, (dihydro)flavin adenine dinucleotide (FAD)[1][2][3][4][5][6][7][8]with galactose-uridine-5’-diphosphate and uridine-5’-diphophate[4]. Reduction of the FAD involves a transformation of the coenzyme from a highly conjugated planar frame to a bent butterfly structure, which induces a conformational change within the enzyme making in more conductive to catalysis, and increases activity rate[2][7]. This flavin reduction also results in the translocation of the mobile loop inward toward the substrate with the Uridine portion of the ligand moving upward toward the flavin[2]. The flavin reduction and corresponding conformational change results in the enzyme being primed for covalent catalysis[2].
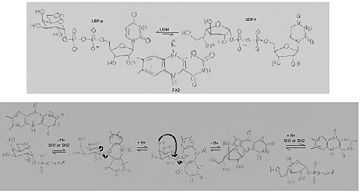
The conversion between UDP-Galp and UPD-Galf involves the cleavage of the anomeric C-O bond, the reaction involves distortion of the ring allowing access to the O4 on C-1 to release the UDP[2][7].
There is a covalent adduct created between the flavin and the substrate that involves a nucleophilic attack (SN1/2) by the nitrogen atom (N5) of the isoalloxazine ring on the substrate which creates covalent falvin-galacrose adduct, that affords the formation of an iminium intermediate in the mechanism, and a displaces the UPD, as a good leaving group, which in turn allows the galactose ring to attach to the flavin, rearrange, dissociate and give the final product[2][5][6]. The C4 of the flavin provides a means to shuttle protons from the C4-OH to the nascent C5-OH after ring opening[2].
The substrate-binding, whose site is adjacent to the flavin cofactor directs the dynamic bridging of a recognition loop with distal FAD, which works to close the recognition loop over the substrate in the active site, and W160 sites, which interact with the Uralic of the substrate and is the N-terminal anchor of the recognition loop[6][8].
Arg174, located outside the putative active site on a mobile loop (recognition loop) adjacent to the putative active site pointing away from the flavin; once the substrate binds it closes this loop and brings the Arg174 side chain in toward the pyrophosphoryl group of the ligand[6]. This ‘recognition’ loop closes over the substrate-binding site, and is there by ‘locked’ down by the Arg174 coordination of the α-phosphate[2][8].
Binding sites and important residues:
The cleft to which the flavin binds, in each monomer, is, adjacent to the substrate binding site and is, lined with conserved residues 167-177: His63, Tyr155, Gln159, Trp160, Try185, Phe186, Arg250, Asn270, Arg280, Tyr314 and Asp351[2][5][6]. These, collectively, have a charge density consistent with binding of a negatively charged substrate such as reduced FAD[5][6][8].
Other residues involved in the conformational change of UGM, once the substrate is bound, are: Phe152, which sandwiches the Uralic ring of the ligand between it’s aromatic ring and that of Tyr155, and Leu175, which is located on the mobile loop moves to be adjacent to the bridging O of the ribose moiety and Phe152[6].
To position the sugar moiety the flavin, Tyr349, Arg280, Asn84, and an ordered water molecule all work together while theArg174 coordinates the α and β phosphates of the UDP, and with Try349 stabilizes the closed loop ‘locking’ it down[2][8].
Arg280 also works to coordinate the α and β phosphates[6][8]. Arg280 is located in the flavin-binding site[6].
This closing of the loop involves residues 167-177 which are adjacent to the Uridine diphosphoryl portion if the ligand and involves a extension of a short helix composed of residues 169-171 encompassing residues 72-174 moving Arg174 in toward the active site[2][6].
Also:
The understanding of the structure of UDP-galactopryranose mutase and the mechanism with which it binds its substrates and catalyzes its reaction is of importance to pharmaceutical drug therapy because UPD-Galactosefuranose and UPD-Galactopyranose are found in many pathogens, in their surface constituents, cell wall glycoconjugates and in a vital component of arabinogalactan that connects peptidoglycan and mycolic acids in myobacteria cell walls in the lipoplysaccaride (LPS) O antigens of some Gram-negative bacteria; but are not found in human/mammal tissues so the this enzyme, UDP-galactopryranose mutase, that interconverts them can be safely inhibited slowing and preventing the growth of pathogenic microbes such as Escherichia coli, Mycobacteria tuberculosis, or Klebsiella pneumoniae[2][4][5][6][7][8].
References:
- ↑ 1.0 1.1 1.2 http://www.brenda-enzymes.org/php/result_flat.php4?ecno=5.4.99.9
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 Gruber TD, Westler WM, Kiessling LL, Forest KT. X-ray Crystallography Reveals a Reduced Substrate Complex of UDP-Galactopyranose Mutase Poised for Covalent Catalysis by Flavin. Biochemistry. 2009 Oct 6; 48(39): 9171-73. PMID:19719175
- ↑ 3.0 3.1 http://www.mondofacto.com/facts/dictionary?UDP-galactopyranose+mutase
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 3int RCSB PDB
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Beis K, Srikannathasan V, Liu H, Fullerton SWB, Bamford VA, Sanders DAR, Whitfield C, McNeil MR, Naismith JH. Crystal Structures of Mycobacteria tuberculosis and Klebsiella pneumoniae UPD-Galactopyranose Mutase in the Oxidized State and Klebsiella pneumoniae UPD-Galactopyranose Mutase in the (Active) Reduced State. J. Mol. Biol. 2005 May 13; 384(4): 971-982PMID:15843027
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 Gruber TD, Borrok MJ, Westler WM, Forest KT, Kiessling LL. Ligand Binding and Substrate Discrimination by UDP-Galactopyranose Mutase. J. Mol. Biol. 2009 Aug 14; 391(2): 327-340. PMID:19500588
- ↑ 7.0 7.1 7.2 7.3 7.4 Zhang Q, Lui HW. Studies of UDP-Galactopyranose Mutase from Escherichia coli: An Unusual Role of Reduced FAD in its Cataysis. J. Am. Chem. Soc. 2000 Sep 27;122(38): 9065-70. DOI: 10.1021/ja001333z
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Yao X, Bleile DW, Yuan Y, Chao J, Sarathy KP, Sanders DAR, Pinto BM, O’Neill MA. Substrate Directs Enzyme Dynamics by Bridging Distal Sites: UPD-Galactopyranose Mutase. Proteins: Structure, Function, Bioinformatics. 2008 June 30; 74(4): 972-79. PMID:18767162
Images:
Images with in the Jmol applet were provided and created by protopedia; the inital view (the defaultt) has provided by protopedia and the green link with in the text (the active site) was created using Jmol within the protopedia applet edit function.
Images (A and B) of protein ribbon portions and important side chains were created using the 3int PDB file from the RCSB Bank in the Swiss PDB Deep-Viewer.
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Joel L. Sussman, Christine Brown, Andrea Gorrell, Alexander Berchansky